Transgenic Drug Discovery
A breakthrough in transgenic mice opens the door to “Humabodies.” Theodora Harold, CEO, and Brian McGuinness, Vice President, Business Development, both from Crescendo Biologics, provide background on the research and what it means for drug discovery.
What’s the story behind Crescendo Biologics?
Crescendo Biologics is a spinout created using IP originally generated at the Babraham Institute in Cambridge, UK, funded by the Biotechnology and Biological Sciences Research Council. Prior to the formation of Crescendo, a research group led by Marianne Bruggemann laid the foundations for highly successful transgenic mice capable of generating fully human antibodies.
Part of what makes mAb-based therapeutics so successful is their superb binding specificity, but constraints imposed by their shape and size mean there are important therapeutic targets and indications that regular mAbs cannot address. Regular mAbs comprise two paired peptide chains – a heavy and light chain (H and L respectively). However, camels and llamas naturally produce a stable class of antibodies that lack the light-chain. These heavy-chain antibodies (HcAbs) contain the smallest unit of an antibody known to recognize and bind to its therapeutic target – the so-called VHH domain. Although camel-derived VHH molecules are a step towards creating the next generation of therapeutics that could address the shortcomings of mAbs, they are not ideal: 1) VHH are not “human” in origin and as such, are likely to be immunogenic when injected into humans; and 2) due to their structural adaptation, VHH are limited in their diversity, reducing the likelihood of successful outcomes in drug discovery.
An important paper published by Marianne’s group in 2007 observed that mice engineered to lack the ability to create antibody L-chains were able to spontaneously, albeit very inefficiently, generate their own HcAbs.
From this concept, Crescendo was born.
What does the work mean for drug discovery?
We’ve built on Marianne’s early work to generate a transgenic drug discovery platform from which fully human, highly diverse antibody VH domains – which we call Humabodies – can be derived.
The Crescendo mouse has been engineered to silence its ability to make regular antibodies and lacks the ability to make antibody light (L) chains. Instead, using a broad range of human antibody H-chain genes, our mouse is engineered to respond to immunization by producing fully human antibody VH domains. The immune response generated by the mouse creates a huge range of different binders with varied binding affinities, recognizing a broad range of epitopes over the surface of the immunizing antigen – which are often inaccessible to the much larger mAbs.
From this pool of building blocks, we believe we can create an almost limitless range of therapeutic molecules optimally configured to engage therapeutic targets. The small size and robust nature of Humabodies allows them to efficiently penetrate tissue, which means they can be formulated for dosing by routes other than injection – topical delivery to the skin, eye, lung and gut, for example. One of our earliest projects was a Humabody antagonist of IL-17A for the topical treatment of psoriasis. This therapeutic encompasses the potency and specificity of systemic mAbs in a molecule delivered locally for local action; however, it has a benign side-effect profile due to low systemic exposure and is, therefore, suitable for chronic use.
We have subsequently focused on cancer with our pipeline of novel T cell enhancing therapies. One of our lead programs is CB307 – a CD137 (4-1BB) x PSMA bispecific that delivers targeted, tumor-specific killing, while avoiding systemic toxicity. It can be applied to a broad range of PSMA-positive cancer indications.
In April 2020, we signed a clinical development partnership with Cancer Research UK to progress another bispecific immunotherapy (CB213) into clinical trials targeting cancers of high unmet medical need. A PD-1 x LAG-3 antagonist, CB213, is a next-generation checkpoint inhibitor designed to deliver safer, more effective therapeutic intervention in patients with cancers resistant to PD-1 blockade alone. Under the terms of the agreement, Cancer Research UK’s Centre for Drug Development will sponsor and fund a future phase I clinical trial for CB213 in patients with solid tumors. We retain the right to further develop the CB213 immunotherapy program by licensing the results of the trial from Cancer Research UK. In preclinical testing, CB213 has demonstrated potent dual checkpoint blockade and the ability to enhance the activity of dysfunctional patient-derived T cells.
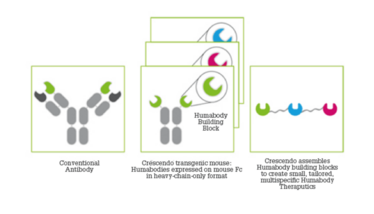
Can you provide more detail on how Humabodies are produced – and the advantages that result?
Because they are produced in vivo in the absence of any antibody L-chains, Humabodies have to “learn” to fold and be stable without a partner L-chain. Thus, the Crescendo mouse acts as a natural filter to produce only VH domains. The processes of somatic hypermutation and in vivo maturation that underpin the immune response generated by the mouse result in the creation of a huge range of different binders with varied binding affinities, recognizing a broad range of epitopes over the surface of the immunizing antigen.
Unlike regular antibodies, and because of their small size and flexible structure, Humabodies can penetrate rapidly into tumor tissue, dispersing homogeneously. By adding a VH domain that binds serum albumin to a Humabody molecule, tumor penetration is enhanced and the circulating half-life of the molecules can be tuned to match the PK characteristics required for a range of different mechanisms of therapeutic intervention.
Regular antibodies rely on their size and the presence of an Fc domain to drive their PK characteristics. However, the presence of an Fc domain can also bring unwanted functional outcomes, for example, because of their engagement of Fc receptors on a variety of cell types causing unwanted target clustering. Humabody-based therapeutics do not contain an Fc domain, unless its inclusion in the design is considered to be therapeutically beneficial.
Humabodies can be used in many ways and, for example, can also be conjugated to form Humabody drug conjugates. Antibody drug conjugates are not simple molecules. They are bulky and can stay in systemic circulation for a long time (often a week or more) without reaching their tumor target. That, coupled with the fact that a large proportion of the toxic payload can simply “fall off” ADCs during that time means that the associated side effects have substantially limited the number of approved ADCs.
Humabody drug conjugates (HDCs) address many of the issues associated with ADCs. Humabody VH domains are small and can penetrate into and accumulate in tumors more rapidly and effectively than bulky, rigid antibodies. And because HDCs can also be tuned to optimize their biodistribution, they don’t spend days circulating systemically. In short, HDCs can carry a smaller amount of a more potent toxic payload and deliver it more effectively to a tumor, resulting in cytotoxic effects within the tumor whilst minimizing exposure of normal healthy tissue to the toxin, enhancing the therapeutic index of the drug.
What are the challenges you face in further developing the platform?
New immuno-oncology therapeutics, such as the checkpoint-targeted mAbs Opdivo and Yervoy, have demonstrated very clearly the power of engaging the human immune system to fight cancers. Indeed, they work exceptionally well as monotherapy, but only in a subset of cancer patients. And when delivered in combination, they can often result in severe, treatment-limiting side-effects.
We are not a big pharma company with a huge pharma research and development budget! So, we need to focus on the indications and patient populations that we think can benefit from our approach. Our Humabody platform is working very well. We have efficient, fast internal processes and a number of T cell enhancing assets in our internal immuno-oncology pipeline. These assets are expected to have an improved safety profile and generate a broad, durable anti-cancer response. We will be filing the clinical trial application for our lead asset, CB307, later this year. We’re also involved in several ongoing collaborations, including one with Takeda, who licensed the first two programs under our global collaboration early, and Zai Lab, who licensed our inflammation program.
It is always a challenge to convince the industry to accept new science and new types of therapeutics, but I think this is slowly changing thanks to the positive outcomes of the forerunners in the antibody fragment space (caplacizumab) and in the bispecific CD137 (4-1BB) field (PRS-343). There are many potential applications for our Humabodies and, although I’m biased, I’m passionate they provide a great starting point for making many drugs that help patients.

"Making great scientific magazines isn’t just about delivering knowledge and high quality content; it’s also about packaging these in the right words to ensure that someone is truly inspired by a topic. My passion is ensuring that our authors’ expertise is presented as a seamless and enjoyable reading experience, whether in print, in digital or on social media. I’ve spent seven years writing and editing features for scientific and manufacturing publications, and in making this content engaging and accessible without sacrificing its scientific integrity. There is nothing better than a magazine with great content that feels great to read."















