Enter the Matrix
My passion for science started early. My father was a scientist, working for a blood transfusion laboratory, and as a hardworking post-doc he used to bring me into the lab with him at weekends or evenings. Before long, he had me making polyacrylamide gels and pipetting samples. I loved it – it was like having the best chemistry kit in the world!
Now I have a lab of my own, I’ve found that the best research comes from finding the right collaborators. My latest project was no exception and started several years ago with a desire to find a better matrix in which to grow the stem cells I work with. Usually as a scientist in a lab, you grow your cells on plastic, which is a completely abnormal environment. I could see that, whilst it gave us some information, it really wasn’t very good at reproducing what we saw in animal models or human studies. So I worked with a team who had developed a promising new hydrogel. Combining their expertise in materials biology with my knowledge of cell and matrix biology, we were able to develop something completely new. Instead of just reacting to the plastic that they’re growing in, the stem cells in this new and improved matrix reacted to each other, and even start to make their own matrix – a much better recapitulation of what goes on within the body.
Having come from a cancer background, I always knew one of the applications of the gel could be in studying the interaction of cancer cells with the local environment. But it took a fortuitous meeting with Gillian Farnie to start the ball rolling. Gillian and I both attended a session on stem cells at Manchester University. I gave a general presentation about hydrogels, and afterwards Gillian approached me. Gillian had been working for a long time on cancer stem cells, in breast cancer, specifically ductal carcinoma in situ. She had been looking at how cancer cells interact with their matrix and how that might affect whether a tumor remains very small and benign or transforms into something malignant. She has used a variety of techniques over the years to do this, including a lab-based alternative using a hydrogel extracted from mouse material. But that hydrogel is very frustrating to use – you rarely get the same result twice because the material changes every time you buy it. We could offer her a hydrogel that would be consistent and which we could “customize” – in other words, add elements into the gel that make it more similar to the human breast.
Manchester University was interested in developing the new hydrogel as a commercial product, and funded us to look at various possible applications, including cancer research. So we had the funding in place to immediately start preliminary experiments. The results from that early work were so encouraging that we’ve now secured further funding to develop much more accurate in vitro models of breast tissue from healthy women, and women with local or metastatic breast cancer. The core of the work is proteomics – essentially, we will identify the most common proteins that make up the breast tissue and use them to “decorate” our standard hydrogel. As a glycobiologist myself, I can’t neglect the sugar component either, so we’re working with a group in Boston who can tell us what kinds of glycans occur in the breast tissue. We’ll also be able to tweak the density of our model breast tissue, which could prove very useful. It is thought that very dense breast tissue might provoke more invasive behavior in tumor cells; that’s hard to study in mice, because their breast tissue is very different to human, and you can only collect retrospective data from women. With our matrix, researchers will be able to observe the behavior of cancer cells in real-time with different matrix densities.
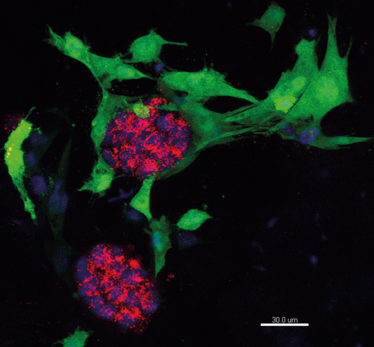
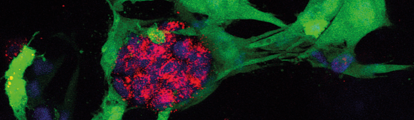
Breast cancer cells growing in the hydrogel. Credit: Gillian Farnie
In general, people tend to think of tissue engineering in terms of regenerative medicine. Undergraduate- level students often assume that I’m going to be teaching them how to make a complete liver or a brain! But actually, if we can just make tiny bits of tissue that behave like they do in the body, it will revolutionize drug development. If you can develop a more effective drug for breast cancer, that’s going to have a bigger impact on a larger number of people than tissue engineering – and at a much lower cost. At the moment, drug screening involves huge numbers of animals, which is not great ethically, but also introduces costs that limit the number of drugs that can be screened. If we had better selection mechanisms, we could screen a lot more drug candidates at that stage. When you show a group of students a little blob of cells in a gel, they aren’t usually quite as excited as when you show them a prosthetic nose, but I always say, “that blob of cells is the future!”
Cathy Merry leads the Stem Cell Glycobiology Group at Nottingham University, UK.
Bargain Brains by Diane Hoffman-Kim
Cathy Merry leads the Stem Cell Glycobiology Group at Nottingham University, UK.















