
Bargain Brains
In some ways, we biomedical engineers are really just control freaks. By designing our own small world in a dish, we can stage manage every aspect and push the boundaries in a way that’s not always possible in an animal model.
In our case, we use in vitro models to study how nerves grow and differentiate in complex environments – with the ultimate goal of treating neurological diseases. Recently, we developed a new in vitro system, made up of small spheres of neural tissue – mini brains. We are not the first to do this; in fact, there are more sophisticated models already out there – but producing them requires a lot of time, expertise and money. Our goal was to create a useful model that an average neurobiology lab could reproduce with minimal investment.
We held ourselves to high standards in terms of the health and viability of the cell culture, and the reproducibility of the method. Very briefly, we take a small piece of rodent brain and isolate the cells, which we seed onto a 3D petri dish, developed by a Brown University colleague, Jeff Morgan. The dish is made up of a material that discourages the cells from sticking to it, so instead they stick to each other. The cells migrate towards each other and assemble themselves into tiny organoids – in this case, mini brains. Within 24 hours we see the cells form a ball of tissue, and within two weeks they make electrical connections. The method is high throughput (from the brain of one rodent we can make 1000 mini brains), simple and cheap. In fact, we didn’t realize just how cheap until our media officer at Brown asked us to quantify it – we did some calculations and were impressed to find that the cost is about $0.25 per brain.
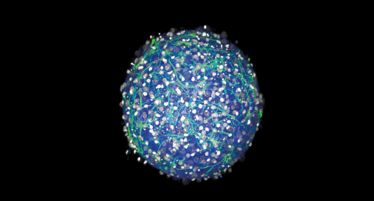
3D mini brains can be produced for just 25 cents each. Credit: Hoffman-Kim lab/Brown University
We have wonderful colleagues here at Brown, which gave us plenty of opportunities to test different aspects of the system. We carried dishes of mini brains to Julie Kauer’s lab upstairs for sophisticated electrical recording, and across the hall to Eric Darling’s lab to test the mechanical properties with atomic force microscopy. We were able to establish that mini brains have many of the same fundamental properties as actual brains. They have the same basic cell types, are electrically active and produce their own matrix. This matrix gives them the same “squishiness” as real brain tissue, which is particularly relevant for research into conditions such as traumatic brain injury. Of course, the mini brains don’t have the complex organization or connectivity to the rest of the body that gives rise to the sophisticated functions of a real brain – this isn’t a B movie and the mini brains can’t think or feel! But they should prove very useful for researchers studying neurobiology or looking for new treatments for brain injury or disease.
In general, I think the future will see more and more of these organoid models being used in biomedical research. Since we published the method, we’ve discussed potential applications with researchers from all around the world. There will always be room for 2D culture, but cells growing in 3D express genes and make electrical connections in ways that more closely mimic their in vivo counterparts. The research community is very excited about the potential of these models.
Diane Hoffman-Kim is Associate Professor of Medical Science and Engineering in the Department of Molecular Pharmacology, Physiology, and Biotechnology, Brown Institute for Brain Sciences, and the Center for Biomedical Engineering at Brown University, USA.
Enter the Matrix by Cathy Merry
Diane Hoffman-Kim is Associate Professor of Medical Science and Engineering in the Department of Molecular Pharmacology, Physiology, and Biotechnology, Brown Institute for Brain Sciences, and the Center for Biomedical Engineering at Brown University, USA.















