The Extraocular Muscles in ALS: A Research Riddle
Could the preservation of the eye muscles in ALS provide a new outlook on a deadly disease?
At a Glance:
- ALS is characterized by progressive loss of motor neurons, leading to complete paralysis. But with few exceptions, the extraocular muscles remain unaffected
- Tjust’s research explored eye muscle involvement in ALS, and the theory that the characteristics of certain cell types, such as satellite cells, play a crucial role in helping the eye muscles remain resilient to the disease
- Preliminary data suggest that there could be further protective mechanisms in place in the eye that potentially shield it from neurodegeneration
- The extraocular muscles are a valuable model system for scientists hoping to better understand ALS through basic research
Amyotrophic lateral sclerosis (ALS) is a devastating condition. An incurable neurodegenerative disease, it is characterized by a progressive loss of upper and lower motor neurons, leading to complete paralysis and eventually death through respiratory failure, usually within three to five years of symptom onset. Currently, the only treatment available is riluzole, but this drug only extends the lifespan of ALS patients by an average of two to three months.
Clinically, the disease can present itself with intriguing variability. It may manifest at any age, but often occurs in people who are middle-aged or older. Subsequent survival is also variable, with some patients surviving under a year, and a small proportion living for decades. When the patient experiences the first symptoms, they are mostly restricted to a single extremity – for example, the right hand. The disease most commonly presents itself distally with a mixture of upper and lower motor symptoms, such as weakness and loss of dexterity. This is later followed by a progression into more proximal muscles, appearance of the same symptoms in the ipsilateral leg, and eventually, involvement of all voluntary muscles in the body. In about 25 percent of the cases, the disease manifests in the bulbar region, first affecting muscles of articulation and mastication. Another variable trait in ALS is the degree of cognitive involvement. Some patients retain all of their premorbid personality and functions, and some patients develop an outright frontotemporal lobe dementia.
ALS and the eye
Despite the various ways in which ALS can present itself, nearly all ALS patients have one thing in common: you don’t encounter them in the ophthalmologist’s office. With some notable exceptions (1), the extraocular muscles are seemingly preserved in most ALS patients, even until the terminal stage. Notably, eye movements and blinking are usually the last modes of communication available to terminal ALS patients (2). But why?
That simple question presents an area of study with huge potential; understanding the underlying mechanisms for eye motility sparing in ALS could provide new insights into how the progress of ALS could be slowed down in more vulnerable muscles.
From an evolutionary perspective, extraocular muscles and their motor neurons are ancient companions that pre-date the advent of terrestrial life on Earth. Extraocular muscles are present and innervated according to a principally similar system in lampreys, whose ancestors (Cambrian cyclostomes) diverted from what would evolve into jawed fish (gnathostomata) between 460 and 535 million years ago. This implies that extraocular muscles are at least that old. The muscles of the trunk, gills and fins of Cambrian fish have since evolved into muscles of terrestrial locomotion, anti-gravity balance, breathing and grasping, but the extraocular muscles still serve (though with greater performance) the same basic function of orienting the gaze, just as they did half a billion years ago.
Exploring the mysteries of eye motility
In my recent doctoral thesis, I explored the sparing of eye motility, using histological studies of the extraocular muscles of ALS patients and the most commonly used mouse model for ALS. Although ALS is a motor neuron disease and therefore frequently studied from the perspective of the central nervous system, skeletal muscles are also important players in its progression. During embryonic development and after the initial establishment of the neuromuscular junction – the specialized synapse that forms between muscle fibers and motor neuron axons – muscle fibers provide the innervating motor neuron with neurotrophic factors, such as glial cell-derived neurotrophic factor (GDNF). The neurotrophic factors are retrogradely transported along the axon back to the nerve cell body in the central nervous system, promoting neuronal survival signaling. The relationship between muscle fibers and motor neurons is critical during embryonic development, where 30–50 percent of all motor neurons projecting to muscles in the limbs and trunk are lost to apoptosis in favor of those motor neurons that are more successful in establishing contact with many muscle fibers. In contrast, muscle fibers in the extraocular muscles appear to provide maturing motor neurons with more generous amounts of neurotrophic factors, and therefore a much larger proportion of motor neurons escape apoptosis – a fact that also explains the exceptionally small motor unit sizes (the relationship between the number of muscle fibers controlled by a single neuron) present in the extraocular muscles.
Studies have shown that deterioration of the contact between muscle fibers and motor neurons, starting at the so-called neuromuscular junction, is an early manifestation of ALS that precedes the actual loss of motor neurons. Therefore, adaptions and maladaptions that take place at the level of the neuromuscular junction could play a large role in the progression of ALS. A good example of this is the observation that when GDNF is overexpressed in the muscles of ALS animal models, it leads to a prolonged survival of the animals, whereas overexpression in glial cells located much closer to the motor neurons in the spinal cord has no such effect. Further examples of this relationship between muscle fibers and motor neurons are conditional knockout mice where ablation of satellite cells, resident stem cells of muscle tissue, leads to impairment in the re-establishment of neuromuscular junction following nerve injury (3).
Asking the Right Questions
The relative resistance of extraocular muscles in the context of ALS, when viewed on its own, seems like a pathophysiological oddity. But if we take a broader view of the numerous degenerative diseases being studied across different areas of medical research, a very different picture emerges.
In different neurodegenerative diseases, monogenetic, dominantly inherited sub-forms of diseases have been identified, where a seemingly ubiquitous (or near-ubiquitous) gene product mysteriously exerts its pathogenic effect mainly on a specific type of cell. For example, whereas hexokinase 1, an enzyme responsible for the phosphorylation of glucose, is present in most tissues in the body, specific mutations in the gene that codes for it can lead either to a dominantly inherited retinitis pigmentosa or, in the case of another missense mutation, a recessive form of Charcot-Marie-Tooth disease. Mutations in the gene coding for superoxide dismutase 1 are responsible for approximately six percent of ALS cases. But despite the pancellular ubiquitous nature of this enzyme (with the highest concentrations actually found in the liver), the mutated form of the protein seems to primarily affect motor neurons in the CNS.
Again, in retinal disease, mutations in the gene coding for bestrophin-1 leads to vitelliform macular dystrophy, a progressive retinal disease that mostly spares the rods. Interestingly, bestrophin-1 seems to be important in chloride ion shuttling in different types of epithelia throughout the body (such as in the airways and colon), not just in the retina. Apparently, in the complex constellation of genes and proteins that sustain our cells, certain cellular processes have become more robust and tolerant to flaws in some cell types than in others.
It is reasonable to look at any disease based on how the patient presents him or herself and ask yourself: “What is wrong with organ X in patient Y?” However, as researchers and physicians, we are looking for solutions to problems. Sometimes we should look for answers from the other end of the tube and ask ourselves, “If this cellular problem is so ubiquitous in my patient, what are cells X and Y doing right that cell Z is doing wrong?”
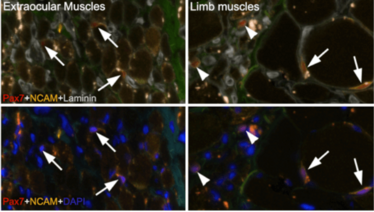
Figure 1. Fluorescence images of a cross-sectioned extraocular muscle (left column) and a cross-sectioned limb muscle (right column) from an ALS patient. Satellite cells (white arrows) and other myogenic stem cells (white arrowheads), are identified by their molecular markers Pax7 (red) and NCAM (yellow) and presented in relation to cellular membranes (grey – top row) and to cell nuclei (blue – bottom row). It seems that satellite cells are still present in both extraocular muscles and limb muscles at the very terminal stage of the disease.
Satellite studies
Satellite cells are present in all muscles of the body, and are normally in a resting state. In response to training or injury, they can become activated, causing them to proliferate and generate new myonuclei for growing and regenerating muscle fibers. Satellite cells in extraocular muscles differ from other satellite cells in several regards. Compared with other satellite cells, they maintain a heightened expression of several developmental transcription factors and have been shown to proliferate and produce new myonuclei more efficiently than limb muscle satellite cells when engrafted into muscle tissue. It has also been proposed that they are more abundant, and in a more continuous state of activation, when compared with satellite cells in limb muscles (4). Interestingly, in vitro studies involving satellite cells from limb muscle biopsies of ALS patients and satellite cells from the most commonly used mouse model for ALS have shown that their growth performance in vitro is impaired compared to satellite cells derived from unaffected patients. Could satellite cells in limb muscles become worn out in a protracted disease course?
In our research group, we asked ourselves – what role might satellite cells play in the resilience of extraocular muscles to ALS? Our results (both published and as of yet unpublished) (5)(6) suggest that the previously reported abundance and continuous activation of satellite cells in extraocular muscles might have been overstated, and that only a small portion of the extraocular muscle, close to the tendon, maintains an increased pool of satellite cells. We found that the majority of the muscle belly contains generally low numbers of satellite cells, and the majority of them are not in an active state. Further analysis of extraocular muscles from ALS patients revealed similar results. Analysis of limb muscles revealed more dynamic changes, with varying numbers of satellite cells in limb muscles of different ALS patients. Importantly, however, satellite cell numbers in ALS patients typically varied between normal and high levels compared with normal elderly sedentary individuals and did not appear to wear out or decrease in numbers during a protracted disease course [Figure 1]. Rather, those muscle fibers in the most severely affected limbs that still retained contact with a motor neuron tended to increase in size together with an increase in the number of satellite cells and myonuclei associated with them. Therefore, it seems that satellite cells, while possibly affected at a level that can be demonstrated in culture conditions, are able to perform well enough in their native niche within the muscle. By extension, the distinguishing traits of satellite cells in extraocular muscles does not appear to be a key element in the sparing of eye motility in ALS.
Making connections
In both the animal model [Figure 2] and in ALS patients, there is a maintained presence of terminal axons at the muscle fiber endplate, in contrast to limb muscles, where large portions of the muscle fibers lose axonal contact (7)(8). Loss of axonal contact is an early manifestation of ALS, which suggests that the protective mechanisms present in the extraocular muscles are influencing disease progression at a relatively early stage. However, my preliminary, unpublished data suggest that not all fiber types in the extraocular muscles are preserved. Slow-type muscle fibers, which in the extraocular muscles are innervated at several points along the length of the fiber rather than at a single point in the middle (as is usual for muscle fibers) appear to be affected by ALS, decreasing in size and proportion in terminal patients. We believe that eye motility is maintained not because of a general sparing of all fiber types in the extraocular muscles, but rather because of compensatory mechanisms elicited by one or several other of the specific fiber types present in the extraocular muscles.
A powerful tool
Other investigators have tried to answer the question of differential vulnerability in ALS by comparing the transcriptomes of vulnerable motor neurons with spared motor neurons. One such study (9) has revealed that oculomotor neurons natively express higher levels of several growth factors. And the factor IGF-II, seems to exert a neuroprotective effect on spinal cord motor neurons cultured in vitro, and when overexpressed through an injected viral vector [Figure 3], extends the lifespan of the most commonly used ALS mouse model by around 10 percent (10). Also, besides the presence of neurotrophic factors and other growth factors, the intense activity in oculomotor neurons has also lead to numerous evolutionary adaptations to sustain the constant activity and stresses of ion cycling that takes place with each depolarization cycle. Such adaptations include a different composition of GABA-receptors with more powerful inhibitory responses, as well as a lessened susceptibility to glutamate excitotoxicity, a mechanism that has been recognized as an important contributor to ALS pathophysiology since the early 1990s.
Future studies will hopefully answer the question of whether the sparing of eye motility in ALS is a result of the summation of many factors that happen to be beneficial in delaying the deleterious processes of ALS, or whether more specific traits present in the oculomotor neurons have the coincidental effect of delaying ALS. Nevertheless, studying selective sparing is a powerful tool for researchers in different fields, as it encourages us to learn from biological systems that already, through pre-existing tools, have a solution to the problems we face.
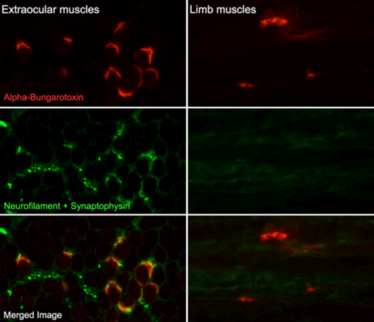
Figure 2. Fluorescence images of neuromuscular synapses, in one extraocular muscle (left column) and one limb muscle (right column) in an ALS mouse model. The contact between motor axon (green) and motor endplate (red) is retained in extraocular muscles (bottom left), whereas the contact is broken and disappears in limb muscles as the disease progresses (bottom right).
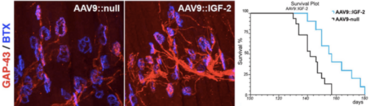
Figure 3. Comparison of motor axons (red) forming synapses with the motor endplate (blue) in limb muscles from two SOD1 ALS mice that were injected after symptom onset with either an empty AAV9-vector (left panel) or an IGF-2-expressing AAV9-vector (middle panel), showing how IGF-2, a growth hormone normally more highly expressed in oculomotor neurons than in spinal cord motor neurons, improves the sprouting capacity of the remaining motor neurons. Mice treated with this vector also exhibit a slower progression rate and survive longer than mice that were treated with the sham vector (right panel). Adapted from (10).
Anton Tjust is a Researching Physician at the Department of Pharmacology and Clinical Neuroscience, Umeå University, and an intern at the University Hospital of Umeå, Sweden.
- H Hayashi, S Kato, “Total manifestations of amyotrophic lateral sclerosis. ALS in the totally locked-in state”, J Neurol Sci, 93, 19–35(1989). PMID: 2809628.
- AR Murguialday AR, et al., “Transition from the locked in to the completely locked-in state: a physiological analysis”, Clin Neurophysiol, 122, 925–933 (2011). PMID: 20888292.
- W Liu et al., “Inducible depletion of adult skeletal muscle stem cells impairs the regeneration of neuromuscular junctions”, eLife, 4, (2015). PMID: 26312504.
- KM Kallestad et al., “Sparing of extraocular muscle in aging and muscular dystrophies: a myogenic precursor cell hypothesis”, Exp Cell Res, 317, 873–885, (2011). PMID: 21277300.
- M Lindstrom et al., “Pax7-positive cells/satellite cells in human extraocular muscles”, Invest Ophthalmol Vis Sci, 56, 6132–6143 (2015). PMID: 26393672.
- AE Tjust, “Extraocular muscles in amyotrophic lateral sclerosis [Summary]”, Umeå University (2017). Available at: bit.ly/tjust-thesis. Last accessed April 25, 2017.
- AE Tjust et al., “Unaffected motor endplate occupancy in eye muscles of ALS G93A mouse model”, Front Biosci (Schol Ed), 4, 1547–1555 (2012). PMID: 22652891.
- JX Liu et al., “Distinct changes in synaptic protein composition at neuromuscular junctions of extraocular muscles versus limb muscles of ALS donors”, PLoS One, 8, e57473 (2013). PMID: 23468993.
- E Hedlund et al., “Global gene expression profiling of somatic motor neuron populations with different vulnerability identify molecules and pathways of degeneration and protection”, Brain, 133, 2313–2330 (2010). PMID: 20826431.
- I Allodi et al., “Differential neuronal vulnerability identifies IGF-2 as a protective factor in ALS”, Sci Rep, 6, 25960 (2016). PMID: 27180807.
Anton Tjust is a Researching Physician at the Department of Pharmacology and Clinical Neuroscience, Umeå University, and an intern at the University Hospital of Umeå, Sweden.















