
The COVID-19 Pandemic: A Summary
Curious about COVID-19? Expert pathologist Fred Plapp summarizes the current state of play…
Download the PDF here for easy reference!
What is a coronavirus?
Coronaviruses are a large family of enveloped, non-segmented, single-stranded, positive-sense RNA viruses that circulate among animals including camels, cats, and bats. Coronaviruses derive their name from their electron microscopic image, which resembles a crown – or corona (see Figure 1).
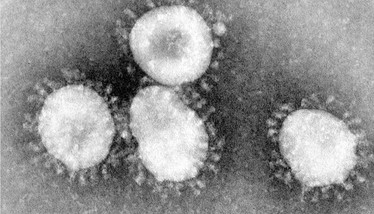
Figure 1. A coronavirus viewed under an electron microscope. Credit: CDC/Fred Murphy.
Six strains of coronavirus have infected humans, four of which are together responsible for about one-third of common colds. In the past two decades, there have been three global coronavirus outbreaks (1). Severe Acute Respiratory Syndrome (SARS), caused by a coronavirus termed SARS-CoV, started in 2003 in Guangdong, China, and spread to many countries in southeast Asia, North America, Europe, and South Africa. Bats are the natural hosts of SARS-CoV; its intermediate hosts are palm civets and raccoon dogs. Early cases of SARS were linked to human and animal contact at live game markets. Transmission occurred person-to-person through droplets produced by coughing or sneezing, via personal contact, and by touching contaminated surfaces. In SARS, peak viral shedding occurs approximately 10 days after the onset of illness, when many patients are hospitalized, which explains why health care professionals have a particularly high risk of becoming infected. SARS-CoV has a R0 of 4, meaning that each infected person spreads the disease to an average of four others, and a case fatality rate of 9.5 percent. Although the virus infected 8,069 persons and caused 774 deaths, the last known case of SARS was detected in September 2003.
Nine years later, MERS-CoV – which causes Middle Eastern Respiratory Syndrome (MERS) – emerged in Saudi Arabia. MERS is characterized by sporadic zoonotic transmission from camels and limited episodes of person-to-person transmission. Explosive nosocomial transmission has been linked to single super-spreaders of infection. Almost all cases have been linked to people in or near the Arabian Peninsula.
The symptoms of MERS are nonspecific, but many patients develop atypical pneumonia and severe acute respiratory distress. Up to 80 percent of patients with MERS require mechanical ventilation. Additionally, patients often have prominent gastrointestinal symptoms and acute kidney failure. This constellation of symptoms is due to the binding of the MERS-CoV S glycoprotein to dipeptidyl peptidase 4, which is present in the lower respiratory tract, gastrointestinal tract, and kidney.
Like SARS, health professionals are at high risk of contracting MERS. The disease is still circulating and, to date, has infected approximately 2,500 people and caused 850 deaths. The main factor that controls the spread of MERS-CoV is its very low R0 of 1. However, the case fatality rate is very high at 35 percent.
On December 30, 2019, a cluster of patients with pneumonia of unknown etiology was observed in Wuhan, China, and reported to the World Health Organization (WHO)’s China bureau in Beijing. By January 2, 2020, the full genome of a new coronavirus (SARS-CoV-2) had been sequenced by Shi Zhengli, a coronavirus expert at the Wuhan Institute of Virology; just over a week later, the sequence had been published and the Chinese National Health Commission warned of its potential danger. The virus was initially referred to as “novel coronavirus 2019” (2019-nCoV) by the WHO – but, on February 11, 2020, was given the official name of SARS-CoV-2 by the International Committee on Taxonomy of Viruses (2).
SARS-CoV-2 is a betacoronavirus (an enveloped, single-stranded RNA virus) that shares 79 percent of its genetic sequence with SARS-CoV and has 96 percent homology with the RATG13 coronavirus strain in bats. However, unlike bat coronaviruses, SARS-CoV-2 has a spike protein optimized for high-affinity binding to human ACE2 receptors and a functional polybasic cleavage site at the junction of the spike protein’s S1 and S2 subunits (a feature that enhances spike protein cleavage and increases viral infectivity).
The virion contains four structural proteins (spike, envelope, membrane, and nucleocapsid) and single-stranded RNA (see Figure 2).
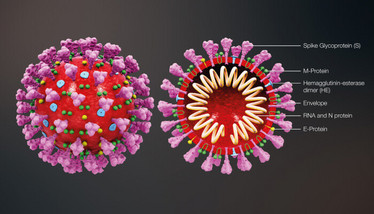
Figure 2. The structure of SARS-CoV-2. Credit: Scientific Animations™.
The RNA genome consists of 29,903 nucleotides – larger than most other RNA viruses. One-third of the genome consists of genes for the four structural proteins and eight genes for accessory proteins that inhibit host defenses. Most of the remainder of the genome consists of the replicase gene, which encodes two large polyproteins that are cleaved into 16 nonstructural proteins (NSP) that assist in replicating and proofreading the viral genome (see Figure 3).
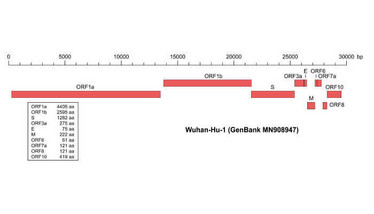
Figure 3. The organization of the SARS-CoV-2 genome. Credit: Wikimedia user Furfur.
SARS-CoV-2 virions attach to human cells with their densely glycosylated spike protein and bind with high affinity to the angiotensin-converting enzyme 2 (ACE2) receptor on human cells. The spike protein is functionally divided into the S1 domain, responsible for receptor binding, and the S2 domain, responsible for cell membrane fusion. Specifically, the RBD of the spike protein mediates recognition of the ACE2 receptor. These receptors are present on many types of cells throughout the body – including lungs, heart, liver, intestines, kidneys, testes, and blood vessels. These cells also possess the TMPRSS2 serine protease, which is needed to cleave the spike protein and facilitate cell entry by SARS-CoV-2.
Once the virus has attached to the ACE2 receptors, the TMPRSS2 protease cleaves the spike protein to expose a fusion peptide. Virions are then able to enter and release their RNA into infected cells, where it is replicated and translated into new viral proteins. Nucleocapsid proteins bind to RNA molecules and are then encapsulated by the envelope, spike, and membrane proteins to form new virions. Infected cells can produce 100 to 1,000 virions per day.
Where did SARS-CoV-2 come from?
How the virus evolved to become transmissible to humans is not known, but two theories predominate: either natural selection in an animal host before zoonotic transfer to humans, or natural selection in a human host after zoonotic transfer.
The first scenario is possible because different coronaviruses infecting the same host can exchange gene segments. A bat virus like RATG13 coinfecting an animal with another coronavirus could have acquired a receptor-binding domain (RBD) more adept at infecting humans, leading to SARS-CoV-2. In this scenario, the pandemic would have emerged rapidly as soon as humans were infected, because the virus had already evolved to become highly infectious.
In the second scenario, a non-pathogenic version of the virus jumped from an animal host into humans and then evolved to its current pathogenic state. For instance, some pangolin coronaviruses have an RBD structure nearly identical to that of SARS-CoV-2. A pangolin coronavirus could have been transmitted to a human; these animals are highly valued in traditional Chinese medicine and sold in markets such as the Wuhan Seafood and Wildlife Market, where many early human cases occurred.
Another, more provocative theory suggests that SARS-CoV-2 was created (accidentally or intentionally) at The Wuhan Institute of Virology, a facility with a long history of bat coronavirus research. These theories suggest that the virus was either intentionally or accidentally released into the surrounding community. Although the lab has researched recombining the genomes of coronaviruses from different species to determine their potential to infect human cells, prominent virologists in the US consider it highly unlikely that SARS-CoV-2 could have acquired both of its unique features (a highly infectious RBD and a polybasic cleavage site) in tissue culture.
This type of research is performed in Biosafety Level 4 (BSL-4) laboratories, which provide the highest level of biocontainment and follow the most stringent biosafety protocols. However, pathogen leakage from BSL-4 labs has been documented on several occasions. The world’s last known case of smallpox was caused by a leak from a British laboratory in 1978; an outbreak of foot and mouth disease in 2007 had a similar origin; laboratories in the United States have accidentally released both Ebola and a deadly strain of avian influenza; and Chinese laboratory workers have been infected with SARS-CoV and transmitted it to outside contacts on at least two occasions. Today, there are approximately 70 BSL-4 laboratories in 30 countries, with more planned. Many scientists fear that, with so many biologists actively hunting for bat viruses and performing gain-of-function experiments, the world is at increasing risk of a laboratory-derived pandemic.
What is COVID-19?
The disease caused by the SARS-CoV-2 virus is known as coronavirus disease 2019 – or COVID-19.
According to the Johns Hopkins Center for Systems Science and Engineering, as of June 26, 2020, there have been over 10.1 million confirmed cases of COVID-19 and 502,589 deaths worldwide (3) – but these numbers are still growing steadily (see Figure 4). Globally, the confirmed case fatality rate is above 5 percent – that is, one in every 20 people with a confirmed positive COVID-19 test has died of the disease.
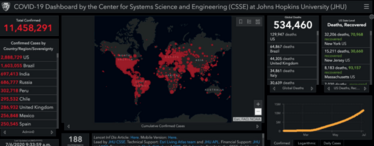
Figure 4. Global COVID-19 cases as recorded by the Johns Hopkins Center for Systems Science and Engineering.
The first US COVID-19 patient was diagnosed in late January. As of June 26, 2020, there have been 2,422,312 confirmed cases of COVID-19 in the country and 124,415 deaths. The average number of new cases per day in the US peaked at 31,000 on April 10, 2020, and then slowly declined to a plateau of approximately 22,000 per day. A few weeks after reopening the economy, however, the number of new cases per day has increased steadily up to 33,000. Current models estimate that between 3 and 10 percent of Americans (between 10 and 33 million people) have been infected so far.
Fortunately, the number of deaths per day in the US has decreased from over 2,000 per day in April to approximately 600 per day in mid-June. The decrease in deaths may be explained by a shift to infections of younger people, continued protection of older people, more testing of people who are asymptomatic or have mild symptoms, and better treatment. Other countries have not experienced this disconnect between the increase in new cases per day and the number of deaths per day – but, because deaths lag behind new cases by approximately three to four weeks, deaths in the US are expected to rise again.
The incubation period before the onset of COVID-19 symptoms ranges from one to 14 days, with a median of 5–7 days. Patients, who have a median age of 59 years, present with fever, dry cough, loss of smell or taste, shortness of breath chills, rigor, fatigue, myalgia, headache, sore throat, and diarrhea.
COVID-19 has a broad clinical spectrum, ranging from asymptomatic infection or mild upper respiratory tract illness to multifocal pneumonia, respiratory failure, and death. Approximately 80 percent of patients experience mild to moderate disease, 15 percent have a severe course requiring intensive care, and 5 percent require mechanical ventilation. Patients may develop pneumonia towards the end of the first week of infection. The mean interval from onset of symptoms to hospitalization is between 9 and 12 days; mean duration from symptom onset to discharge from the hospital is 25 days.
The most severe cases develop pneumonia and acute respiratory distress syndrome (ARDS). Vital signs predictive of a severe course include respiratory rate over 24 breaths per minute, heart rate over 125 beats per minute, and oxygen saturation over 90 percent on room air.
The most common abnormal laboratory findings include lymphopenia (70 percent), increased CRP (61–86 percent), mildly prolonged prothrombin time (58 percent), elevated lactate dehydrogenase (40 percent), elevated AST and ALT (4–22 percent). Results that predict severe disease include D-dimer >1000 ng/mL, ferritin >300 μg/L, lactate dehydrogenase >245 IU/L, absolute lymphocyte count <800, neutrophil-to-lymphocyte ratio >3, platelet count <35,000/μL, CRP >100 mg/L, and elevated high-sensitivity troponin.
Cytokine release syndrome (“cytokine storm”) is caused by the overproduction of early-response proinflammatory cytokines, including tumor necrosis factor, IL-1β, and IL-6 (one of the best biomarkers of cytokine storm severity and mortality risk).
Chest radiographs are abnormal in 60 percent of cases (77 percent if severe). Chest CT is abnormal in 86 percent of cases (95 percent if severe). Chest X-rays are characterized by bilateral patchy infiltrates and chest CT scans demonstrate ground-glass opacities in 86 percent of cases. There is a peripheral distribution in over 50 percent of cases. “Crazy paving” and consolidation become the dominant CT findings (see Figure 5), peaking 9 to 13 days, followed by slow clearing (4).
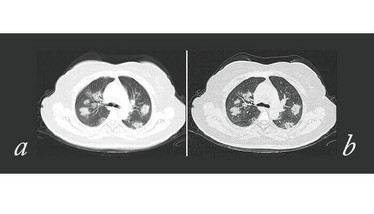
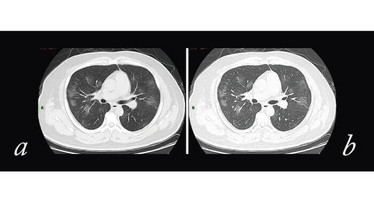
Figure 5. CT imaging of COVID-19. Top: a) Thin layer CT and b) high-resolution CT showing multiple patchy and light consolidation in both lungs of a 50-year-old woman with COVID-19. Bottom: a) Thin layer CT and b) high-resolution CT showing multiple patches and “crazy paving” in both lungs of a 38-year-old male with COVID-19.
Risk factors for COVID-19 include:
- older age
- ethnicity
- male gender
- comorbidities (including hypertension, diabetes, coronary artery disease, chronic lung/kidney/liver disease, cancer, hematologic malignancy, organ transplant, or immunosuppression)
People with underlying health conditions are six times more likely to be hospitalized and 12 times more likely to die from the disease compared with patients who had no pre-existing conditions.
Approximately 30 percent of hospitalized COVID-19 patients develop progressive pulmonary disease. The major cause of COVID-19 mortality is respiratory failure secondary to ARDS and thrombosis. ARDS is characterized by leakage of fibrin-rich fluid from pulmonary capillaries into alveoli. It may be caused by direct binding of SARS-CoV-2 to ACE2 receptors, which regulate the production of angiotensin, on endothelial cells. Impairment of ACE2 activity may lead to activation of the kallikrein-bradykinin pathway, which in turn increases vascular permeability. Infected endothelial cells also express leukocyte adhesion molecules that recruit activated neutrophils and lymphocytes to the site of injury. The accumulation of cytokines, neutrophils, and lymphocytes causes inflammation, loosens endothelial cell junctions, increases vascular permeability, promotes alveolar fluid retention, and enhances pulmonary tissue damage.
A recent autopsy report compared the histologic patterns of lungs from patients who died from influenza with patients who died from COVID-19. Both groups had diffuse alveolar damage with hyaline membranes and perivascular T-lymphocyte infiltrates. The lungs from COVID-19 patients had distinctive vascular features due to SARS-CoV-2 invasion of endothelial cells, including disruption of cell membranes and severe endothelial injury. This caused microangiopathy and widespread thrombosis in the small vessels and capillaries of the lungs. Alveolar capillary microthrombi were nine times more prevalent in patients who died from COVID-19 than in those who died from influenza.
Recent reports from Europe and North America have described clusters of children and adolescents requiring admission to intensive care units with Pediatric Inflammatory Multisystem Syndrome (PIMS) associated with SARS-CoV-2 infection. The syndrome has many overlapping features with Kawasaki disease. Thus far, children have been given anti-inflammatory treatments, including parenteral immunoglobulin and steroids.
Early identification and testing of individuals with COVID19 symptoms have been the primary focus of public health mitigation. However, many studies have shown that a significant proportion of individuals infected with SARS-CoV-2 do not have any symptoms at the time of testing.
Infection rates vary widely between populations (see Table 1). However, in all studies, the proportion of individuals who were symptom-free when they tested positive was consistently high. Because these studies tested circumscribed populations, the percent of people who are asymptomatic and test positive is likely overestimated. Some experts suggest that the asymptomatic rate is 40 to 45 percent.
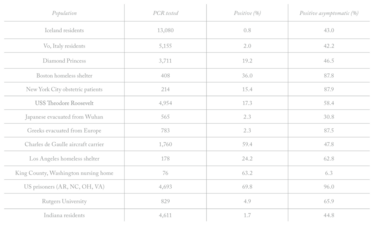
Table 1. A summary of studies on COVID-19 testing results, highlighting the percentage of positive test results obtained from asymptomatic patients.
Asymptomatic patients have the same viral load as many symptomatic ones and can transmit the virus for at least 14 days. And the absence of symptoms in people infected with SARS-CoV-2 does not mean that they are free from harm; of the asymptomatic individuals who had lung CT scans, 33–48 percent had ground glass opacities.
The risks of COVID-19 are highest in older patients with pre-existing conditions such as hypertension, diabetes, cardiovascular disease, and obesity. The common theme? Their association with vascular inflammation and endothelial dysfunction. Multiple studies have noted a higher incidence of pulmonary embolism and venous thromboembolism in COVID-19 patients admitted to the intensive care unit.
SARS-CoV-2 may directly infect endothelial cells by binding their ACE2 receptors. Endothelialitis – infection of endothelial cells and subsequent perivascular inflammation – disrupts vascular integrity, exposes capillary basement membranes, and activates the coagulation cascade. Activated endothelial cells express P-selectin, von Willebrand factor (VWF), and fibrinogen, which enhances platelet adhesion. Activated platelets release VEGF, which triggers endothelial cells to upregulate the expression of tissue factor. Together, these events lead to thrombosis and tissue ischemia.
An imbalance of VWF and ADAMTS13 may also play a role in COVID-19-associated coagulopathy. VWF is an acute phase response protein released by activated endothelial cells in response to inflammatory stimuli. In the event of vascular injury, VWF facilitates binding of platelets to sub-endothelium through its interactions with collagen, inducing thrombus formation. VWF also binds to neutrophil extracellular traps and recruits platelets and leukocytes to promote thrombosis. In contrast, ADAMTS13 is a negative acute response protein; its activity decreases in response to inflammation. Release of proinflammatory mediators during the severe phase of COVID-19 may increase the secretion of ultra-large VWF multimers that are not completely cleaved (due to decreased secretion of ADAMTS13), which can lead to thrombosis and thrombocytopenia. In response to blood clot formation, the fibrinolytic pathway is activated (which explains the high levels of D-dimer and its close correlation with poor patient outcome).
SARS-CoV-2-induced thrombosis may be responsible for the very low oxygen saturation levels in some patients. COVID-19 reduces tissue oxygenation not only by causing pneumonia and ARDS, but also by promoting thrombosis.
How is COVID-19 transmitted?
Two factors facilitated the initial rapid spread of COVID-19 in Wuhan: i) a population of 11 million inhabitants that increased the chance of person-to-person contact, and ii) the city’s busy transportation hub, which increased the likelihood of exporting cases to other locations. Despite Chinese containment measures, COVID-19 has grown into a full-blown pandemic.
The R factor, a virus’ basic reproductive number, is referred to as R0 – the average number of people someone carrying the virus will infect. The higher the R0, the faster an epidemic can spread. At the start of the pandemic, R0 for SARS-CoV-2 was estimated at 2.0 to 2.5, indicating that one patient could transmit the virus to two (or slightly more) other people. The doubling time for COVID-19 cases is estimated at three to six days.
Figure 6. A transmission electron microscopic image of an isolate from the first US case of COVID-19. Credit: CDC.
The virus (see Figure 6) is transmitted primarily through droplets 5–10 μm in diameter, released when an infected person coughs, sneezes, talks, or even exhales. These airborne droplets can attach to the respiratory tract mucosa or conjunctiva of another person. They can also settle on surfaces or fomites and be transferred to another person upon contact. SARS-CoV-2 is more stable on plastic and steel (up to three days) than on cardboard (up to one day) or copper. Viral transmission is possible if someone touches their face, eyes, nose, or mouth following contact with contaminated surfaces or fomites.
Transmission may also occur through aerosols, which are particles smaller than 5 μm. SARS-CoV-2 remains viable in these particles for up to three hours. Aerosol transmission is a serious risk to health care workers during procedures such as intubation, bronchoscopy, suctioning, turning a patient to the prone position, or disconnecting a patient from the ventilator.
Some experts estimate that exposure to as few as 1,000 SARS-CoV-2 particles can cause infection. One releases about 3,000 respiratory droplets that travel at 50 miles per hour; most are large and quickly fall to the ground, but many remain airborne and can travel across a room in a few seconds. A sneeze releases about 30,000 droplets traveling up to 200 miles per hour, most of which are small and travel great distances. A single cough or a sneeze emitted by an infected person may spread as many as 200 million virus particles.
In contrast, a single breath releases only 50 to 5,000 droplets, most of which travel at low velocity and drop quickly. Because breath is expelled at low force, viral particles residing in the lower respiratory areas are not expelled – meaning that breathing may release as few as 20 to 30 viral particles per minute. Speaking increases the release about tenfold (200 virus particles per minute), so five or more minutes of face-to-face conversation could lead to infection.
But infection with SARS-CoV-2 depends not only on dose, but also exposure time. If an infected person coughs or sneezes directly toward someone, they can inhale 1,000 viral particles in a few minutes. If someone enters a room shortly after an infected person coughs or sneezes, it may take only a few breaths – whereas if they simply occupied a room where an infected person was breathing, it might take 50 minutes or longer to inhale an infectious dose.
The mean duration from symptom onset to death is 18 days. Case fatality rate (CFR), which is calculated by dividing the number of deaths by the number of known cases, has been reported at 6.4 percent worldwide – significantly higher in older patients. But CFR almost certainly overestimates the true lethality of the virus. The number of confirmed cases usually includes only people whose symptoms were severe enough to be tested, resulting in a severity bias. Epidemiologists estimate there are five to 10 times more people with asymptomatic infections. Additionally, the number of deaths may be inaccurate at the time of calculation because deaths typically occur one to two months after a person becomes infected and not all deaths are apparent at the same time. COVID-19 deaths that occur at home are underreported compared with those that occur in a hospital.
The infection fatality rate is the proportion of infected people who will die from COVID-19, including those who do not get tested or become symptomatic. The infection fatality rate is estimated to be between 0.5 and 1 percent. Even at this rate, COVID-19 is a serious public health threat. For comparison, the infection fatality rate of seasonal influenza is approximately 0.1 percent – and it nevertheless kills hundreds of thousands of people each year.
All 3,711 passengers and crew members aboard the Diamond Princess cruise ship were tested and 19 percent were positive for COVID-19. The case fatality rate, which included only symptomatic individuals, was 2.6 percent. However, 47 percent of passengers who tested positive were asymptomatic at the time of testing. The infection fatality rate, which included both symptomatic and asymptomatic individuals who tested positive, was 1.3 percent.
If one assumes that the number of asymptomatic or minimally symptomatic cases is several times as high as the number of reported cases, the case fatality rate may be less than 1 percent. Even though its case fatality rate is lower than MERS-CoV, SARS-CoV-2 will cause many more deaths, because there have been – and will continue to be – so many more cases. As with other coronaviruses, health care-associated transmission appears to be a major mode of infection.
One histopathological study of the lungs of a deceased patient reported the presence of hyaline membrane formation (see Figure 7), interstitial mononuclear inflammatory infiltrates, and multinucleated giant cells. These findings were consistent with acute respiratory distress syndrome.
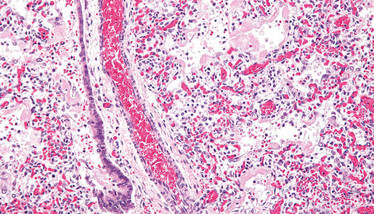
Figure 7. Hyaline membrane formation in diffuse alveolar damage, the histological correlate of acute respiratory distress syndrome. Credit: Wikimedia user Nephron.
SARS-CoV-2 RNA has been detected in several body fluids including 93 percent of bronchoalveolar lavage fluid, 72 percent of sputum, 63 percent of nasal swabs, 32 percent of pharyngeal swabs, 29 percent of feces, and 1 percent of blood samples. No urine specimens have tested positive. The average cycle threshold for all specimens was 31, which corresponded to a viral load of less than 2.6x104 copies/mL. Nasal swabs had a cycle threshold of 24, indicating a much higher viral load of 1.4x106 copies/mL.
About 12 percent of COVID-19 patients have gastrointestinal symptoms and 41 percent shed viral RNA in their feces. The presence of viral RNA does not necessarily indicate the presence of live virus, but raises the possibility of human-to-human transmission by the fecal-oral route. SARS-CoV-2 has also been detected in human breast milk from a single mother on days 10, 12, and 13 after birth. Detection of viral RNA coincided with mild COVID-19 symptoms and a positive PCR in the newborn.
CDC guidelines state that routine BSL-2 laboratory practices are adequate for specimens from patients that may have SARS-CoV-2 infection, with the exception that potentially infectious specimens from these patients should be manipulated only in a biological safety cabinet. The CDC explicitly recommends against viral culture from specimens that may contain SARS-CoV-2. However, clinical laboratory staff should wear personal protective equipment (PPE) and implement standard, contact, and airborne precautions, including the use of masks and eye protection. Health care workers should maintain social distancing in the workplace.
How does COVID-19 RT-PCR testing work?
The sequence of SARS-CoV-2 was published by Chinese scientists on January 11, 2020; the following week, virologists in Berlin, Germany, produced the first reverse transcriptase real-time polymerase chain reaction (RT-PCR) diagnostic test for COVID-19. This test was supplied to the WHO and many countries adopted it. Unfortunately, the US Centers for Disease Control and Prevention (CDC) refused to employ this test and prevented laboratories from producing their own assays. On February 5, the CDC began shipping its own SARS-CoV-2 RT-PCR kit – but it produced unreliable results and was deemed unusable. Although the problems were raised on February 7, more than 50 days passed before the CDC developed an alternative test. Even after kits became available, testing was hampered by a shortage of RNA extraction reagents and nasopharyngeal swabs.
Eventually, the CDC published primers, probes, and protocols. The US Food and Drug Administration (FDA) issued new guidance on February 29, 2020, so that labs could develop and use COVID-19 molecular diagnostic tests (but had to apply for Emergency Use Authorization, or EUA, within 15 business days of clinical use). Although clinical labs could purchase primers and probes for the CDC assay from Integrated DNA Technologies (IDT), other reagents had to be procured elsewhere. To remain in FDA compliance, labs had to follow the exact specifications under which the EUA was granted. If they ran the IDT kit on an alternative platform, new EUA approval was required – an ordeal too onerous for most hospital clinical laboratories.
The WHO’s RT-PCR assay targets the SARS-CoV-2 envelope gene and the RNA-dependent RNA polymerase gene. If both targets are detected, the result is reported as positive; if only one is detected, the result is reported as inconclusive. The CDC’s original assay included three different amplification regions of the N gene; NS3 was designed to detect all SARS-like coronaviruses, whereas the N1 and N2 regions were specific for SARS-CoV-2. The NS3 target produced too many false positive results and had to be eliminated.
Other laboratories and diagnostic companies designed RT-PCR assays that targeted various combinations of the open reading frame, envelope, nucleocapsid, and RNA-dependent RNA polymerase genes. The limit of detection of most such assays was 100 viral copies/mL or higher. Several commercial reference labs began testing during the second week of March, courtesy of major in vitro diagnostic vendors with EUAs for their assays, but validations for these assays used synthetic RNA sequences spiked into respiratory samples and data documenting their clinical diagnostic performance was limited.
Recent studies indicate that SARS-CoV-2 viral load peaks in the first five to six days of disease onset. Viral RNA can be detected during the second week of disease onset, but viral load is lower. Despite high sensitivity, a negative PCR is insufficient to exclude SARS-CoV-2 infection in patients with a high pretest probability of infection; timing of specimen collection, specimen source, specimen quality, and method performance affect the accuracy of results. If repeat PCR testing is warranted, the second specimen should be performed at 24 hours after the first collection; longer intervals between specimens increases the risk of missed diagnosis because viral load decreases with time. Repeat testing of a lower respiratory sample (bronchoalveolar lavage) might be necessary for diagnosis of a patient with severe or progressive disease and repeat negative results with nasopharyngeal swabs.
Nasopharyngeal swabs, not throat swabs, should be submitted for RT-PCR testing. If a nasopharyngeal (NP) swab is not inserted properly, there is a higher likelihood of a false negative result. NP swabs should be transported to the laboratory in universal transport media or saline, not sent by pneumatic tube, and should be stored at 2–8°C.
Viral loads measured on NP specimens are much higher in patients with severe disease than in patients with mild disease, although RT-PCR testing may return positive results even in some asymptomatic patients. Most patients with mild disease test negative by 10 days after symptom onset. In contrast, patients with severe disease shed virus for a median of 20 days post-symptom onset. Virus may be continuously detectable until death in some non-survivors.
Serologic tests detect antibodies that form in blood after SARS-CoV-2 infection. To increase availability, the FDA permits companies to develop and distribute serology tests if they validate the tests with specimens from confirmed COVID-19 patients and notify the FDA of their intent. Results must be accompanied by a statement: “This test has not been reviewed by the FDA.”
Over 200 manufacturers have begun marketing serologic tests in the US. Most of the tests flooding the market are lateral flow assays; laboratory-based antibody tests are either enzyme-linked or chemiluminescent immunoassays. Worldwide, concerns have been expressed about the reliability of tests that have been rapidly developed and marketed without rigorous oversight. Some companies claim high sensitivity and specificity without accompanying data, and the FDA has warned that some companies have falsely claimed FDA approval. SARS-CoV-2 antibody tests marketed prior to or without an EUA are not FDA-authorized and have not received a CLIA categorization. These tests are considered high-complexity by default until they receive an approval that permits them to be considered moderate-complexity or CLIA-waived.
Currently available tests target antibodies to one of two SARS-CoV-2 proteins, either the nucleocapsid phosphoprotein or the spike protein. Most lateral flow assays detect IgG and IgM antibodies separately. Enzyme-linked and chemiluminescent immunoassays detect either total antibody, IgG alone, or IgG and IgM separately. There is no substantive advantage to assays that detect IgG over total antibody.
The Infectious Disease Society of America (IDSA) recently published their guidelines on COVID-19 serologic testing (5). They state that antibody testing has not been clinically verified and should not be used as the sole test for diagnostic decisions. Antibody test results should not be used to make staffing decisions or decisions regarding the need for PPE until more evidence about protective immunity is available.
According to the IDSA, SARS-CoV-2 serology may:
- support the diagnosis of COVID-19 in patients who present late and have a negative PCR result, or when lower respiratory tract sampling is not possible
- identify people with an antibody response to serve as convalescent plasma donors
- allow epidemiologic studies of disease prevalence
- verify vaccine response once antibody correlate(s) of protection are identified
Antibody tests should not be used to diagnose acute COVID-19 infections. Individuals with symptomatic COVID-19 generally do not have detectable antibodies to SARS-CoV-2 within 10 days of symptom onset. Most hospitalized patients with confirmed viral RNA have detectable IgG antibodies 14 days after symptom onset; IgM antibodies become detectable only one to two days earlier – so these tests miss infectious patients in the early stages of disease and patients with mild symptoms (who may produce lower antibody titers). They can also fail in elderly or immunocompromised patients, who may not develop detectable levels of antibodies after infection. Even more worrisome, some patients continue to shed viral RNA after seroconversion. A negative serologic test might give patients a false sense of security, leading to reckless behavior.
Seasonal coronavirus antibodies decline only a few weeks after infection and some people are susceptible to reinfection within one year.
Antibody tests may play a role in detecting unrecognized past infection and immunity, but that role must be rigorously evaluated. Currently, no one knows how long antibodies to SARS-CoV-2 persist. Seasonal coronavirus antibodies decline only a few weeks after infection and some people are susceptible to reinfection within one year. More encouragingly, SARS-CoV antibodies peak approximately four months after infection and protect patients for two to three years.
The presence of SARS-CoV-2 antibodies does not guarantee immunity. It is not currently known which antibody responses – if any – are protective or sustained. Of COVID-19 patients who developed antibodies during hospitalization, one in three lacked antibodies that neutralized virus in plaque growth assays (the standard test for antibody effectiveness). The best way to investigate immunity is to follow people with and without antibodies to determine whether they become reinfected.
SARS-CoV-2 seroprevalence remains low even in the most severely affected communities. When overall disease prevalence is low, maximizing specificity and positive predictive value is preferred. An antibody test with specificity of 99 percent or greater will yield the highest positive predictive value in populations with a prevalence of 5 percent or higher. This test has an even greater chance of being accurate when testing a high-risk group, such as exposed healthcare workers or family members.
When should serologic test results not be used? They should not contribute to decisions about grouping people in schools, dormitories, or correction facilities; about returning people to work; or about changes to clinical practice or the use of PPE.
How is COVID-19 treated?
The care of patients with COVID-19 is similar to that of patients with other viral pneumonias. It consists primarily of supportive care and oxygen supplementation when needed. Dexamethasone has been reported to decrease the mortality rate of patients with severe respiratory illness (6). Remdesivir, a nucleoside prodrug that inhibits transcription of many RNA viruses, may shorten COVID-19-related hospital stays by an average of three days (7). Tocilizumab, a monoclonal antibody to IL-6, is being trialed in patients with cytokine storm and severe respiratory disease. Additionally, lopinavir/ritonavir (Kaletra), a mixture of two HIV protease inhibitors, is under investigation. Recently, China approved the use of favilavir (Favipiravir), an antiviral drug used for influenza, as an investigational therapy for COVID-19 (8).
Hydroxychloroquine, much touted for its potential therapeutic effect, was shown in 2002 to interfere with SARS-CoV entry into cells – but does not benefit patients with COVID-19. The FDA recently revoked the emergency authorization of hydroxychloroquine and chloroquine to treat COVID-19 because neither drug demonstrated benefits that outweighed the risks of dangerous cardiac arrhythmias.
On March 24, 2020, the FDA approved the investigational use of convalescent plasma, which contains antibodies to SARS-CoV-2, for patients with serious or life-threatening disease. COVID-19 convalescent plasma (CCP) is a potentially safe and effective, but unproven, therapeutic modality for COVID-19. The FDA requires clinical application of CCP to be conducted under one of three defined pathways: i) an investigational new drug (IND) application to support research – the traditional approach for clinical trials; ii) an emergency use IND for compassionate use in an individual patient with severe or immediately life-threatening COVID-19; or iii) a government-led initiative providing expanded access program (EAP) IND to participating institutions under a master treatment protocol with modest data-reporting requirements. Mayo Clinic leads the EAP IND for hospitalized patients with severe or life-threatening COVID-19.
Several trials have been proposed to evaluate CCP for:
- post-exposure prophylaxis among adults with close contact exposure to COVID-19 who have not yet manifested symptoms
- treatment of patients with confirmed mild disease
- treatment of moderately ill, hospitalized patients who have not been admitted to the intensive care unit admission or required mechanical ventilation
- rescue therapy for patients requiring mechanical ventilation
- safety and pharmacokinetics in high-risk pediatric patients.
Potential CCP donors must meet the following criteria:
- Documented evidence of COVID-19 diagnosis by PCR at the time of illness or positive SARS-CoV-2 antibody test after recovery if PCR was not performed.
- Symptom-free for at least 28 days prior to donation, or at least 14 days prior to donation and a negative nasopharyngeal swab by PCR.
- Compliance with all blood donor eligibility requirements.
Donors are tested for blood type and infectious diseases. Women who have been pregnant are also tested for HLA antibodies; if present, their plasma is not used for transfusion. Each unit of CCP contains approximately 200 mL and should be ABO compatible with the recipient. The dose is one to two units per patient. Transfusion rate is 100 to 250 mL per hour or per hospital policy. CCP units can be frozen and stored for one year; after thawing, plasma may be stored for up to five days at 1–6°C. Possible adverse events are the same as other plasma components. At the moment, CCP is for investigational use only.
When will there be a vaccine?
On May 1, 2020, the US federal government launched “Operation Warp Speed” to deliver a COVID-19 vaccine by January 2021, years ahead of standard vaccine timelines. Usually, a vaccine is first tested in animals. If it appears safe and effective, then the three phases of human clinical trials begin. Phase I evaluates the safety of the vaccine in humans; some vaccines can make a viral infection more virulent by a mechanism called antibody-dependent enhancement. Phase II establishes the formulation and doses of the vaccine to optimize its effectiveness. Phase III then tests the safety and efficacy of the vaccine in a larger group of people. Only after a vaccine passes all three phases is it licensed, manufactured, distributed, and administered to humans. Because of the urgency of the pandemic, some scientists propose replacing this method with faster “challenge trials,” which deliberately expose vaccinated volunteers to the virus and could determine a vaccine’s effectiveness in weeks instead of years.
As many as 123 different SARS-CoV-2 vaccine candidates are under development worldwide, 10 of which are in human trials. Many have not been tested in animals. In July, the National Institutes of Health (NIH) will begin randomized phase III trials to determine if any of these 10 vaccines prevent SARS-CoV-2 infection. They plan to enroll 20,000 individuals who will receive a vaccine and 10,000 who will receive a placebo.
Conventional vaccines rely on the production of either live attenuated virus or inactivated virus. Live attenuated vaccines use a weakened form of the virus to produce an immune response without causing serious illness. Because they use live virus, these vaccines need extensive safety testing. Some live viruses can be transmitted to other people, which is a concern for people who are immunocompromised. Inactivated virus vaccines use a killed virus, which may be safer, but often produces a weaker immune response. These vaccines require multiple doses and boosters to provide long-term immunity. Some vaccines also require adjuvants to enhance the immune response – and work is already underway on licensed adjuvants for use with COVID-19 vaccines.
A vaccine that targets the SARS-CoV-2 spike protein should theoretically prevent the virus from binding to human cells and reproducing. The advent of genetic engineering may allow scientists to produce novel vaccines that specifically target this antigen. A gene for a single SARS-CoV-2 protein can be introduced into cell cultures, which synthesize large quantities of relatively pure protein to serve as a vaccine. Alternatively, the gene can be inserted into an innocuous virus, such as adenovirus, which is then injected into people. The genetically engineered adenovirus infects human cells, replicates, and expresses the spike protein to prompt an immune response.
Some companies are attempting to produce nucleic-acid vaccines, in which a gene for a SARS-CoV-2 antigen is introduced directly as a segment of either DNA or RNA. Such vaccines should carry less risk of contamination, because they do not require cultured cells or viruses, but no RNA or DNA vaccine has ever been licensed for use in humans anywhere in the world. DNA plasmid vaccines transfer the genetic blueprint for RNA into cells, which then synthesize spike antigens; one such vaccine was developed for MERS, but never manufactured. RNA vaccines eliminate the need for DNA plasmids by embedding RNA into lipid globules that can merge with cell membranes. Human cells then synthesize the corresponding antigen. RNA vaccines may produce more potent immunity than DNA plasmids, but they are less stable and must be stored frozen.
Stopping COVID-19 – suppression or mitigation?
Some countries attempted to reduce the infectivity of the pandemic to R0 by enforcing suppression. An R0 below 1 indicates that each infected person transmits SARS-CoV-2 to less than one other person. Successful suppression requires early and widespread testing – including of people without symptoms. Those who positive are isolated so that they cannot infect others.
A failure to implement early testing in other countries has forced them to rely on mitigation, rather than suppression, to slow the spread of disease. Mitigation efforts include handwashing, school and business closings, travel limitations, mask wearing, and social distancing to decrease the likelihood of person-to-person transmission. Mitigation focuses on protecting the most vulnerable from the effects of a disease that is already widespread throughout the community. By reducing the number of active cases at any given time, health care providers can respond without becoming overwhelmed (see Figure 8).
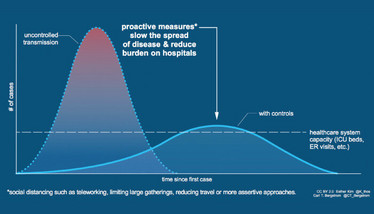
Figure 8. “Flattening the curve,” a mitigation approach to lower and delay the epidemic peak. Credit: Esther Kim and Carl T. Bergstrom.
The steep, dotted curve represents the occurrence of cases over time without protective measures. The flatter, solid peak illustrates the beneficial effect of mitigation – also known as “flattening the curve.” The Center for Infectious Disease Research and Policy (CIDRAP) has predicted that the COVID-19 pandemic will last for 18 to 24 months and will not be halted until 60 to 70 percent of the population becomes immune (10). Depending on control measures, cases may come in waves of varying impact and at different intervals as illustrated by the following three scenarios.
Scenario 1:The first wave of COVID-19 (spring 2020) is followed by a series of repetitive smaller waves that occur through the summer and then consistently over a one- to two-year period, gradually diminishing in 2021. These waves may vary geographically and may depend on what mitigation measures are in place and how they are eased. Depending on the height of the wave peaks, this scenario could require periodic reinstitution and subsequent relaxation of mitigation measures over the next one to two years.
Scenario 2:The first wave of COVID-19 is followed by a larger wave in late 2020 and one or more smaller waves in 2021. This pattern will require the reinstitution of mitigation measures in the autumn to decrease the spread of infection and prevent healthcare systems from being overwhelmed.
Scenario 3: The first wave of COVID-19 is followed by a slow burn of ongoing transmission and case occurrence, but without a clear wave pattern. Again, this pattern may vary somewhat geographically and may be influenced by the degree of mitigation measures in place in various areas. This scenario would likely not require the reinstitution of mitigation measures, although cases and deaths will continue to occur.
Whichever scenario the pandemic follows, a significant level of COVID-19 infection is likely to continue worldwide, with hotspots popping up periodically in diverse geographic areas. As the pandemic wanes, it is likely that SARS-CoV-2 will continue to circulate in the human population and will synchronize to a seasonal pattern with diminished severity over time, as with other less pathogenic coronaviruses.
- J Guarner, “Three emerging coronaviruses in two decades”, Am J Clin Pathol, 153, 420 (2020). PMID: 32053148.
- C Del Rio, PN Malani, “COVID-19 – new insights on a rapidly changing epidemic”, JAMA, [Epub ahead of print] (2020). PMID: 32108857.
- Center for Systems Science and Engineering at Johns Hopkins University, “Coronavirus COVID-19 Global Cases” (2020). Available at: bit.ly/3b6cQWl.
- Z Xu et al., “Pathological findings of COVID-19 associated with acute respiratory distress syndrome”, Lancet Respir Med, [Epub ahead of print] (2020). PMID: 32085846.
- IDSA, “Infectious Diseases Society of America Guidelines on the Diagnosis of COVID-19” (2020). Available at: bit.ly/2NE3SpB.
- P Horby et al., “Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report” (2020). Available at: bit.ly/2BTL6rB.
- JH Beigel et al., “Remdesivir for the treatment of COVID-19 – preliminary report”, N Engl J Med, [Epub ahead of print] (2020). PMID: 32445440.
- Pharmaceutical Technology, “Favilavir approved as experimental coronavirus drug” (2020). Available at: bit.ly/2NAYvaK.
- US Food and Drug Administration, “Coronavirus (COVID-19) update: daily roundup, March 24, 2020” (2020). Available at: bit.ly/2Vt9IP3.
- Center for Infectious Disease Research and Policy, “COVID-19: the CIDRAP viewpoint” (2020). Available at: https://printabletemplates.com/cidrap-covid19-viewpoint/















