Science in the Time of Cholera
The recent FDA approval of Vaxchora could fill an important niche in cholera prevention. We speak with Myron M. Levine, a professor at the University of Maryland School of Medicine, who played an integral role in the development of the vaccine.
Why focus your work on vaccine research?
Since my student days, I’ve been interested in what we now call “global health”. My first involvement with research was as a senior medical student in the 1960s, when I worked for three months on a project studying diarrheal diseases at the Jinnah Children’s Hospital in Karachi. My appetite for research in tropical pediatrics whetted, on my way home from Pakistan I stopped off in what is now Bangladesh (then East Pakistan) and spent some time at the Cholera Research Laboratory in Dhaka, now known as the International Center for Diarrheal Disease Research, Bangladesh.
My post-graduate training in infectious diseases was mentored by John Robbins, one of the developers of the Haemophilus influenzae type b (Hib) vaccine, and I did my military service obligation in the Commissioned Corps of the U.S. Public Health Service as an Epidemic Intelligence Service Officer at the Centers for Disease Control. I spent the following almost half century in vaccine development, though I love clinical work and still enjoy making clinical rounds at some of our overseas sites on occasion. It’s important to keep in touch with the patients and families we are trying to help.
I set up the Center for Vaccine development (CVD) in 1974 and directed it for 40 years, stepping down in 2014 to become Associate Dean for Global Health, Vaccinology and Infectious Diseases, which essentially gives me a license to pursue all the research interests that are on my “bucket list”.
What is the ethos of the Center for Vaccine Development?
Our mission from the start has been to address infectious diseases that afflict impoverished populations in developing countries but are rare in industrialized nations, and therefore get little funding or attention. We tried to fill a niche as a public sector research entity that tries to do everything a small vaccine company would do, other than manufacture and market the product. That includes clinical and laboratory research, epidemiology, biostatistics, regulatory affairs, and so on – everything that is needed to create candidate vaccines, move them through clinical trials, and find industry partners to commercialize them at an affordable cost. From the beginning, we limited ourselves to one major disease area – bacterial enteric infections, such as cholera, shigellosis (bacillary dysentery), typhoid, and various types of diarrhea-causing Escherichia coli.
The Center came into existence at an exciting time for vaccine research. Just one year after its inception saw the advent of recombinant DNA technology, which came to be a very important tool for us. At the same time, advances were being made in clinical research, such as formalized institutionalized review boards (ethical committees) and innovative clinical trial designs.
Is cholera on the rise?
It fluctuates. Cholera is one of the very few true pandemic bacterial diseases. We are currently in the seventh cholera pandemic in recorded history, which began in the early 1960s. During the pandemic, the disease has periodically lain quiescent for a few years, before exploding again, typically in new geographic areas.
What has become clear in recent years, as diagnostic capabilities have improved, is that cholera is a much bigger problem than previously appreciated. In 2013, the CVD reported results from a large Bill and Melinda Gates Foundation-funded project looking at the etiology and burden of moderate-to-severe diarrheal diseases in children in sub-Saharan Africa and Asia – the Global Enteric Multicenter Study (GEMS). We enrolled 22,568 children under five years of age and looked for over 40 pathogens. In all South-Asian sites we were somewhat surprised by the relative importance of Vibrio cholerae as a pathogen responsible for moderate-to-severe pediatric diarrhea.
What led to the discovery of the vaccine?
In the 1980s, we were asked by the NIH to set up a model of cholera challenge in order to test a vaccine intended to stimulate antitoxin antibodies to neutralize cholera toxin. A field trial had given disappointing preliminary results and the NIH wanted us to test the vaccine in naïve individuals as well as study the immune response in depth.
We found that the toxoid vaccine against cholera toxin had conferred only a very modest protective effect, and most of our student volunteers developed cholera diarrhea. This led me to wonder what level of protection Mother Nature herself provided. We asked our earlier volunteers to return and re-challenged them with cholera – there was complete protection and we could not grow V. cholerae in direct stool cultures of the re-challenged subjects, suggesting that anti-bacterial immunity was playing the key role in protection rather than anti-toxin immunity. We continued this research, including re-testing some challenged volunteers up to three years after their first cholera diarrhea and showed that the protection persisted. We concluded that the best approach to prevent cholera would be a live, attenuated vaccine. This was the early days of recombinant DNA technology and there were methods available to delete genes, thereby creating precise attenuations. By the mid-1980s we had developed a strain of V. cholerae O1 that looked very promising, with a deletion in the A (enzymatically active) subunit of the cholera toxin (CVD 103-HgR) and insertion of a mercury resistance gene marker in the gene encoding Hemolysin A.
At a Glance
International non-proprietary name (INN): Cholera Vaccine, Live, Oral
Brand name: Vaxchora
Previously marketed as: Orochol, Mutacol, Orochol E
Developed by: Center for Vaccine Development, University of Maryland
Marketed by: PaxVax (previously manufactured by the Swiss Serum and Vaccine Institute [SSVI])
Drug class: Live-attenuated vaccine
Approval status: Approved by FDA for US adults traveling to cholera-affected regions in June 2016. (Note - The SSVI formulations were previously approved in Switzerland, Canada, Argentina, Australia and New Zealand).
What happened next?
The live, attenuated vaccine was shown to be well tolerated and effective in clinical trials and was licensed to the Swiss Serum and Vaccine Institute, a company based in Berne, Switzerland. Unfortunately, the 1990s were a tough time for vaccine companies and, to cut a long story short, production of the vaccine was halted for financial reasons in 2004. A decade passed before the engineered vaccine strain was picked up again by PaxVax, Inc, whose philosophy is not dissimilar to the CVD. They develop vaccines that fulfill a global health need, achieving commercial viability by first targeting the traveler’s market. The vaccine, Vaxchora™, was approved by the FDA in June 2016 and recommended by the Advisory Committee on Immunization Practices for adults traveling to cholera-affected regions. And CVD and PaxVax have already started to look at applications in the developing world using an appropriate formulation for that purpose (slightly higher dose).

On Virgin Soil
Six years on, Haiti’s cholera epidemic isn’t over yet – and neither is the political fall-out.
Ten months after the 2010 earthquake that saw hundreds of thousands displaced into temporary camps, Haiti was struck by a devastating cholera outbreak. Cholera has since infected an estimated 800,000 (seven percent of the population) and killed around 10,000.
Cholera can kill within hours if left untreated, and death rates were particularly high in the early stages of the outbreak (1). Death from cholera is a result of dehydration and shock, caused by severe diarrhea – most deaths could be prevented with inexpensive oral rehydration salts (2). Cholera had never before been reported in Haiti, so people were unaware of the danger and the need for prompt rehydration treatment – with tragic results. The incubation period for the disease can be as short as two hours, and aid agencies were left playing catch-up as it spread like wildfire.
In 2016, Haitians are still suffering with cholera. However, dedicated cholera treatment centers and access to rehydration therapy have cut the death toll, while education and sanitation efforts have curbed transmission. A vaccination program administering Shanchol to 100,000 people has also shown good efficacy – around 65 percent (3) – demonstrating an important role for reactive vaccination programs in controlling cholera outbreaks.
The impact of the outbreak has not just been medical; the fact that cholera is an “imported” disease has provoked anger. Within a week of the onset of the outbreak, locals pointed the finger at a United Nations base near the Artibonite River, used as a source of drinking water by most of the first victims. Although the UN persistently denied the reports, resentment towards the agency persisted, and distrust slowed the implementation of control measures.
Only in August 2016, after a slew of evidence that peacekeeping troops were the most likely source of the outbreak, did the UN acknowledge their own role in the outbreak. The agency faces legal action from victims who claim that the UN failed to screen soldiers or ensure adequate sanitation at the camp.
How did you feel when you found out that the vaccine would be withdrawn?
The experience taught me to think downstream and take into account business factors from the start. For the first two decades of my career, I was very naïve about the business and financial aspects of bringing a vaccine with very low profit margins to market and making it sustainable. Most big pharmaceutical companies will only consider vaccines that will return their investment within a year or two, which rules out vaccines against many of the infections found in the developing world. That wasn’t widely appreciated by researchers during the 1990s – we thought “if we build it, they will come”. In reality, high development costs and regulatory hurdles meant that many vaccine companies in the developed world were struggling, while very few manufacturers in the developing world were able to produce high-quality vaccines. The advent of Gavi (the Global Alliance for Vaccine and Immunization), launched in 2000, dramatically changed the market landscape; we are now in a much improved situation with respect to the global supply of vaccines and in providing immunization for the world’s most disadvantaged populations.
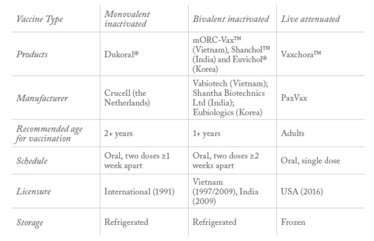
Table 1. Cholera vaccines compared.
How hard is it to recruit volunteers to be infected with cholera?
Perhaps surprisingly, not hard at all. Even in our early studies back in the 1980s, when there was no real precedent for human challenge trials, we were able to recruit volunteers. When we ask our volunteers about their motivation, we find that they are driven by two things. First, the thrill of doing something that is unusual, and that makes them feel good about themselves. Second, since they were modestly compensated (at the same daily rate as someone on jury service in Montgomery County, MD, where NIH is located), they made plans for use of their compensation. Volunteers often told me that they intended to use the money on activities like mountain climbing, skate boarding, white water rafting – things that I would consider much more dangerous than taking part in our studies. Young people exude an air of invincibility!
It probably helps that most of our volunteers don’t feel seriously unwell – cholera mainly manifests in copious watery diarrhea, and with adequate rehydration therapy there is little malaise, nausea, or pain. I often give the example of one volunteer who developed quite severe diarrhea and required intravenous fluids. Despite this, he felt well enough to spend most of his time on the Research Isolation Ward playing ping pong, while still hooked up to his IV drip. Every so often, he would disappear to the bathroom, proceed to the nurses’ cubby to provide his specimen and then go right back to his ping pong game.
Did your human experimental challenge model lead to regulatory challenges?
There is a lot of discussion at the moment about how more frequent use of human challenge models could diminish the costs of bringing vaccines to licensure. As the first FDA approval based on data from challenge trials, the precedent set by our model could help others follow the same path, and we were aware of being “under a microscope”. However, the FDA was very supportive and realistic throughout the process. Previously, there was no FDA-approved cholera vaccine, and with the Haiti epidemic still ongoing not far from the Florida coast, many US citizens visiting affected regions, and our armed forces frequently deployed on missions in the developing world, there was a clear demand.
How is Vaxchora different to existing cholera vaccines?
Our aim is in no way to replace the currently available oral killed vaccines. Existing vaccines are effective for populations who are immunologically primed, preventing seasonal outbreaks of cholera in known hotspots. But for children, or others who have yet to be exposed to the bacteria, they offer much lower protection, and require two full doses to be effective. Live vaccines like Vaxchora achieve correlates of protection much faster than killed ones – and with a single dose. That makes Vaxchora suitable for its current indication as a traveler’s vaccine, and we believe it could also help to limit so-called “virgin soil” epidemics, like the one in Haiti in 2010.
These epidemics occur in populations who have not previously experienced cholera, but where the conditions favor fast transmission. As was seen in Haiti, the first few weeks or months of such an outbreak often see high mortality and morbidity, with healthcare systems struggling to respond to the new threat. In these circumstances, an easily administered single-dose vaccine is required and we hope that Vaxchora can fill that need.
In 2000, when the vaccine was still being manufactured by the Swiss Serum and Vaccine Institute, there was an outbreak of cholera on the Micronesian island of Pohnpei. The logistics made a two-dose vaccination schedule unfeasible, so the WHO asked the company to provide 40,000 doses of the live attenuated vaccine for a reactive immunization program. The vaccine was very successful, with an estimated efficacy of 79 percent. To see Vaxchora being used to limit future outbreaks around the world would definitely be a career highlight.
- D Sontag, “In Haiti, global failures on a cholera epidemic”, The New York Times, 31 March (2012). nyti.ms/2btZJVP
- WHO Factsheet on Cholera, bit.ly/1p2wvsD
- LC Ivers et al., “Effectiveness of reactive oral cholera vaccination in rural Haiti: a case–control study and bias-indicator analysis”, Lancet Glob Health 3, 2162–e168 (2015). bit.ly/2bLQYpr
Myron M. Levine is a Professor of Medicine at the University of Maryland School of Medicine















