No Biomarker, No Trial?
The vast majority of experimental cancer drugs fail during the later stages of clinical development – after considerable time and expense has already been invested. The right biomarkers could help us weed out unsuitable candidates early on, and focus our efforts on therapies with real potential.

The way we have developed cancer drugs to date is far from optimal and, in most instances, results in failure (1). The stark truth is that the majority of drug discovery and development activity yields little benefit to patients, while exposing them to potentially toxic drugs and wasting billions of dollars in the process. With the majority of experimental cancer drugs falling down at the costly mid-to-late stages of clinical development (Phase II or III clinical trials), we’ve reached a stage where something has to give. We need to bring critical decision points forward, ideally into the initial stages of clinical development (Phase I), before costs, timelines and patient numbers escalate. The shrewd application of biomarkers in early-phase clinical development can help us to make these critical go/no-go decisions in time.
Biomarkers come in many flavors
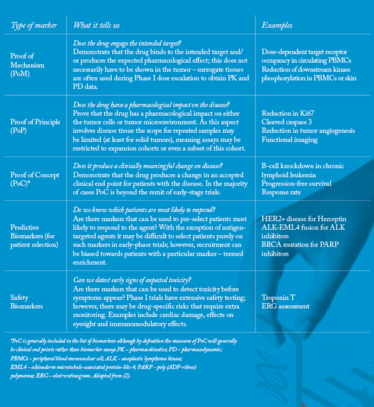
A biomarker is defined by the FDA as “a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.” Under the umbrella term of “biomarkers”, there are many different flavors. One of the most commonly adopted biomarker classification systems is that suggested by Bradley (2), which categorizes biomarkers (with some minor modifications) as follows:
- Pharmacodynamic
- Proof of mechanism (PoM)
- Proof of principle (PoP)
- Proof of concept (PoC)
- Predictive biomarkers (sometimes known as patient stratification, selection or enrichment biomarkers)
- Safety biomarkers
These biomarker categories are explained in more detail below.
Biomarkers to the rescue?
Biomarkers can provide us with data to make critical development decisions in early-phase cancer trials in at least two important areas.
Confirming the biological effect
The use of PoM and PoP pharmacodynamic biomarkers allows an early assessment of pharmacological activity of a new drug. Traditionally, dose-finding first-in-human oncology trials have relied on escalating the dose of the drug up to a maximum tolerated dose (MTD; the highest dose of a drug or treatment that does not cause unacceptable side effects), which is then declared the recommended dose for further development. For many (if not most) emerging oncology therapies, dosing to MTD is either impractical or nonsensical. The assumption that higher doses correlate with therapeutic effect, which is inherently linked to toxicity, may be appropriate for “traditional” cytotoxic drugs but not for many of today’s molecularly targeted agents.
In the absence of desirable “off-target” pharmacology, dosing beyond a relevant pharmacodynamic plateau is likely to offer little benefit but instead risks increasing toxicity or even producing confounding effects. Rather than blindly escalating the dose to the MTD, applying appropriate PoM pharmacodynamic biomarkers during the dose escalation stage of a clinical trial can provide an estimated optimum dose, without having to expose patients to unnecessary toxicities. PoP biomarkers can then be examined in patient expansion cohorts treated with the identified dose, in order to confirm the pharmacological impact on the tumor.
Selecting the right patients
Biomarkers that identify patients most likely to respond to a given therapy are known as predictive biomarkers. At the most basic level, molecularly targeted agents and therapeutic IgG antibodies will not work in tumors that lack the relevant target/antigen or are not reliant on that target for survival; for example, where simultaneous blockade of two pathways is required to cause cell death (3). Well-known examples include:
- Herceptin won’t work in tumors not expressing HER2;
- Inhibitors of PARP have a greater effect on tumors with existing deficiencies in DNA repair, such as BRCA1/2 mutations;
- The ALK inhibitors crizotinib and ceritinib have no efficacy in non-small-cell lung cancer patients lacking EML4-ALK translocations.
The use of predictive biomarkers has the potential to make clinical trials smaller, shorten development timelines and avoid exposing non-responsive patients to unnecessary toxicity. As a result, they are becoming a cornerstone in patient therapy. Even so, we must remember that, while attractive, markers of sensitivity or resistance may be challenging to establish in early trials and can be more complex than expected. For example, at least five subtypes of EML4 ALK are now known, each with different sensitivities to crizotinib, which can prove challenging when screening large numbers of patients for these rare markers.
Best practice for biomarkers
We’ve established that biomarkers can bring forward critical go/no-go decisions, and make the drug development process more efficient by allowing candidates to “fail early and fail fast”. But there are a host of practical considerations to take into account before deciding when and how to make use of biomarkers in a given trial.
Biomarker development should begin as early as possible, ideally at the target validation stage, and continue throughout early drug discovery and beyond. It is particularly important to establish the key pharmacokinetic and pharmacodynamic relationships well ahead of a clinical trial. For example:
- Concentration required to produce a pharmacological effect in vitro;
- Duration of the biological effect during and after administration of the drug in vitro;
- Administered dose, schedule and plasma concentration needed to produce biological effects in vivo;
- Any toxicological changes that could be detected by adding safety biomarkers into preclinical studies and/or the clinical trial.
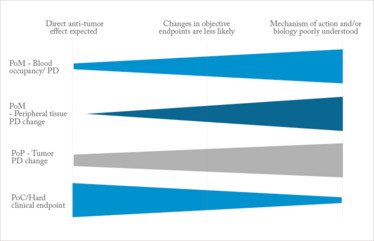
Figure 1. One size doesn't fit all. Shows the relative importance of different biomarker types, depending on the properties of the drug being tested.
Without an understanding of these elements, the clinical dose and schedule cannot be selected with any certainty.
During biomarker assay development, consideration must be given at all times to the four “S”s:
- Science – is there a scientifically relevant biomarker we can use?
- Suitability – can the assay reliably detect the pharmacodynamic change we expect to see clinically?
- Study design – do we know when to take a clinical sample and how many we will need to provide a statistically robust decision?
- Sample – can we actually deliver this assay in the real world?
Particularly for first-in-class trials, it can be difficult to define a scientifically relevant biomarker, as the underlying biology may not be adequately characterized. Although it might be possible to generate a potential biomarker empirically in vitro or in vivo, without a thorough understanding of the underlying target biology, translation of the biomarker to the clinic will always be hazardous and the results difficult to interpret. It is also essential to gain a thorough understanding of assay performance prior to a clinical trial; close attention must be paid to analytical validation of assays. While assessing the sensitivity of an assay is relatively achievable, understanding what magnitude of change is biologically relevant in patients is much more challenging. Indeed, the clinical utility of a new biomarker may need to be investigated at the same time as the drug being tested, which makes setting critical study go/no-go decision criteria related to changes in pharmacodynamics very challenging, especially in the early stages of drug development.
During preclinical development, identifying when the peak pharmacodynamic effect is observed (both after a single dose and over repeated dosing) is essential, preferably in the context of the drug pharmacokinetics achieved. Establishing appropriate sampling times based on pharmacodynamic results during a Phase I trial is impractical; however, it is feasible to use preclinically established pharmacokinetic/pharmacodynamic relationships to focus on appropriate time points in the clinic.
An oft-neglected aspect of biomarker development is sample integrity. Even the most established assay cannot produce reliable information from a degraded sample. Assays of live cells, phosphoproteins, mRNA and metabolites are generally the most challenging in this regard. Ensuring consistent sample handling between multiple sites, with subsequent analysis at a single site, may be necessary to make sure biomarker results are comparable between trial patients.
The problem with predictions
While in many instances suitable predictive markers may not be available or required for a first-in-human trial, such markers may be appropriate or even vital for further development of the agent. There are several preclinical and clinical approaches that can be taken to identify such markers, but they generally fall into three groups:
- Rational selection – choosing potential predictive biomarkers based on known biology and then testing the hypothesis in appropriate cell lines and preclinical in vivo cancer models.
- Screening-based selection – looking at the efficacy of the agent across a wide panel of cell lines, such as the Wellcome Trust Sanger “Genomics of Drug Sensitivity in Cancer” project (http://www.cancerrxgene.org/), and selecting potential predictive biomarkers based on the genotype of sensitive cells lines. This can be based on single or multiple genes; although in this latter case testing the hypothesis experimentally can be very challenging.
- Retrospective response analysis – collecting biopsy material from patients during a trial and retrospectively genotyping to differentiate a predictive signature based on responders and non-responders. This is very challenging in early-stage clinical trials with small patient numbers but tumor material can at least be collected for future analysis.
In cases where a single characterized tumor marker is required (for example, monoclonal antibodies and small molecules targeting specific mutations), patient selection is relatively straightforward. However, for many agents a single, simple marker for efficacy is not available and in these cases it may not be possible or even advisable to select patients in early-phase trials.
Where a predictive biomarker is available, in the vast majority of cases it will necessitate the analysis of tumor tissue, which in itself can be a challenge. While existing historic biopsy material is relatively easy to obtain, each patient’s disease evolves over time and with treatment, so historic tissue may not reflect the current status of the tumor. Historic tissue also tends to be formalin fixed, which can limit the utility of antibodies in patient screening. In contrast, a contemporary biopsy taken as part of the clinical trial will be far more relevant to the patient’s disease and can be processed as needed. The downside is that the requirement for biopsies as part of trial inclusion may severely limit recruitment. This is especially true where a relatively small proportion of the patient population is expected to be positive for the biomarker and most biopsies will therefore prove futile.
The decision on the use of biopsies for patient selection should be made on a case-by-case basis according to the biology of the target, the design of the clinical trial, and the intended patient population. In addition, successful patient selection or enrichment can depend on the assay type, reagents used, preparation of tissue and performance of the assay. Therefore, defining a positive result for inclusion across a range of patients, assays and trials can be very challenging. Decisions on setting “positivity” limits to entry criteria based on patient selection markers should be made on a case-by-case basis with reference to both the known biology of the target and, if possible, additional data on the patient population to be investigated (from biobanks, for example). A balance needs to be struck between setting limits too high to achieve recruitment or too low to get any biologically meaningful data. Finally, for any trial intending to use predictive biomarkers, it is vital to understand the size of the patient population – and the logistical challenges associated with screening early on – to allow a rational discussion on the clinical viability of the project.
One size doesn’t fit all
Unfortunately, it is impossible to have a single prescriptive biomarker strategy for all possible oncology drug classes and targets; there is just too much diversity to allow this. Even so, provided the four “S”s are adhered to, a biomarker approach can be developed for any cancer trial.
We must remember that, fundamentally, clinical trials are experiments conducted to answer a scientific question about a particular therapy and/or the underlying tumor biology. Given this, the biomarkers chosen for an early-phase clinical trial will be highly dependent on the nature of the scientific and clinical aims of the trial, type of agent, etc. There are many ways in which this might be considered but, at the Cancer Research UK Centre for Drug Development, we generally use four broad categories as a reference framework:
1. Clinical trials in which a direct, quantifiable anti-tumor effect is expected
Such trials might include cytotoxic agents, synthetic lethal combinations, antibodies with anti-tumor antibody-dependent cellular cytotoxicity or antibodies armed with cytotoxic or other therapeutic payloads. The emphasis in these trials is on PoP and objective clinical markers of PoC such as response rate or progression-free survival. The use of predictive biomarkers may or may not be required, depending on the mechanism of action of the agent. Cell-based immunotherapies, such as chimeric antigen receptor technologies, may also fall into this category given that they have direct anti-tumor effects and have been shown to be effective in treating advanced, hard-to-treat cancers.
2. Clinical trials in which direct changes in objective clinical endpoints are less likely
Here, agents may target tumor cell migration, metastasis or angiogenesis. Agents in this class will generally have PoP biomarkers that are well characterized, making the detection of any biological effect of the agent comparatively straightforward. Patient selection for such targeted agents is likely to become very important. In the absence of a clinical response, peripheral PoM biomarkers to guide dose selection are likely to be necessary, as well as PoP in tumor to gain evidence for further development.
3. Clinical trials in which the mechanism of action and/or downstream biological effect on the tumor are poorly understood
Examples here might include agents targeting tumor-associated immune cell phenotypes or tumor metabolism. Unlike the category above, the purpose of such a trial is likely to be focused as much on exploring the mechanism of action of the agent, as advancing its development. In extreme cases the aims of the trial may be entirely related to hypothesis testing and the agent used purely as a tool with no inherent “developability”. In these cases both PoM and PoP biomarkers become necessary to fulfil the aims of the trial but suitable assays are unlikely to have been characterized clinically or even exist preclinically. Predictive biomarkers are unlikely to be possible. In these cases a very comprehensive preclinical assessment of potential clinical biomarkers is required before a decision can be made on whether to translate a project to the clinic. These studies are likely to be a clear-cut case of “no biomarker, no trial”.
4. Therapeutic cancer vaccine trials
These tend to fall outside the above approaches as simple pharmacokinetic/pharmacodynamic relationships do not strictly apply and the definitions of PoM and PoP are more concerned with the development of an anti-target immune response than a direct anti-cancer response per se. PoC clinical efficacy readouts such as progression-free or overall survival might be considered for such trials depending on the patient population being studied. Demonstrating clinical benefit in an advanced cancer population is unlikely, but may be expected in a newly diagnosed population with low tumor burden.
Biomarkers for all?
The aim of early phase clinical trials should be to establish if an agent warrants further clinical development or to robustly test a scientific hypothesis in the clinic. If a biomarker is needed to meet the aims of a clinical trial, performing the trial without an appropriate biomarker is futile. Overall, the situation is more complex than “No Biomarker, No Trial” and requires a firm understanding of what a clinical trial is aiming to achieve and how exactly biomarkers can support this aim. Nevertheless, rationale application of biomarkers in early clinical development can stop an agent from becoming another victim of mid- to late-stage cancer drug failure. Perhaps it would be more accurate to say “No Biomarker Strategy, No Trial”.
James Ritchie is a drug development scientist, Sidath Katugampola is a biomarker development specialist and Paul Jones is a preclinical sciences manager at Cancer Research UK Centre for Drug Development, London, UK.
- M Hayet al., “Clinical development success rates for investigational drugs”, Nature Biotech, 32, 40–51 (2014).
- E Bradley, "Incorporating biomarkers into clinical trial designs: points to consider", Nature Biotech, 30, 596–599 (2012).
- WG Kaelin Jr., "The concept of synthetic lethality in the context of anticancer therapy", Nature Rev Cancer, 5, 689–698 (2005).
James says that he wants to live in a world where the word “cancer” no longer holds any fear, and he has dedicated his professional career to achieving this aim, having been involved in cancer drug discovery and development since 2001. His experience has spanned the entire continuum from early discovery through to pivotal clinical development and he is currently the drug development scientist at Cancer Research UK’s Centre for Drug Development. The remit of the CDD is the translation and early clinical development of new anti-cancer agents covering everything from small molecules to immune and cellular based therapies.
Growing up in a family of medical professionals, I soon realized that doing on-call at nights did not fit in with my love for a good work–life balance. More importantly, it also made me question how medicines work and why they often work better for some people than others. This curiosity led me to study Pharmacology at Southampton University. I received a Ph.D from the University of Cambridge and spent the next 10 years working at Pfizer UK, six of those years working in Biomarkers and Translational Medicine. I have been fortunate to carry on with this passion over the last four years at Cancer Research UK and continue looking answers to my childhood questions.
Paul has a lifelong fascination for biology, studying genetics and gaining a PhD in molecular toxicology before embarking on a series of post-doctoral positions on a variety of receptor signalling mechanisms. On gaining an R&D position in GE Healthcare Medical Diagnostics he became a passionate born-again drug developer. For the last nine years Paul has pursued this interest at Cancer Research UK on the translation of novel small molecules, biologicals, cell therapies, imaging agents and radiotherapies into first-in-man clinical trials.















