Breaking the Back of Breakbone Fever
Developing a dengue vaccine has been like a 20-year game of Snakes and Ladders – but in this game, there are four billion winners, as the prize could help protect half the world’s population. Here, we talk to Bruno Guy (Associate Director, Dengue Scientific Affairs) and Marie-José Quentin-Millet (formerly Vice-President Product Conception & Development) about the long and complex history of Sanofi Pasteur’s Dengvaxia.
At a Glance
International non-proprietary name: Dengue Tetravalent Vaccine (Live, attenuated)
Brand name: Dengvaxia
Previous name: CYD-TDV
Developed by: Sanofi-Pasteur
Marketed by: Sanofi-Pasteur
Drug class: Biological
Approval status: For the target population, during the 25 month active phase, Phase III trials showed an efficacy against virologically confirmed dengue of 65.6%, against severe disease of 93.2% and against hospitalization of 80.8% (1, 2). Following this, Dengvaxia was approved for use in individuals aged 9 to 45 years in Mexico, Philippines, and Brazil (Dec 2015) and in El Salvador (Jan 2016). The submission process is continuing with additional approvals expected.
Why did Sanofi Pasteur decide to focus on dengue?
Marie-José: It’s always important to start with the why! In a way, the reason is simple – dengue threatens roughly four billion people worldwide. At present, about 128 countries are affected, but that could increase as climate change and international travel expand the geographical range of the vector. In fact, it’s the fastest-growing mosquito-borne disease in the world. And it’s not like malaria, which is caused by a protozoal parasite that you can treat with drugs, and where the mosquito vector mainly bites at night so you can protect yourself with bed nets. There’s no specific treatment for dengue. Furthermore, the vector bites throughout the day, and staying inside is no protection, because the main vector (Aedes aegypti) really likes humans – and really likes their homes. People are afraid for themselves and their families and we wanted to stop that.
Bruno: As a company, you could say we had personal experience of the disease. When the project started in 1992, few people in Europe had heard of it, but it was supported by our then CEO, Alain Audubert. He himself had suffered with dengue, so he knew how bad it can be, and perhaps that helped foster an understanding of the importance of the disease in Sanofi Pasteur (“Pasteur Merieux Serums et Vaccins” at that time). And he wasn’t the last Sanofi Pasteur employee to get dengue, by any means – only in the past year, two of our colleagues from the dengue team based in Lyon, France, got dengue while traveling in Asia and had to be hospitalized.
How did you get started?
Bruno: Dengue is a complex disease, comprising four different virus serotypes, D1–D4. People can be infected with any serotype in any order – there’s no real cross-protection against the wild type infections. So an effective vaccine needs to protect people against all four serotypes at once, which is a very challenging requirement. We understood from the very beginning that the nature of this challenge demanded the participation of leading scientists wherever they might be, so we set up a global network of collaborations with external laboratories and dengue experts. These kinds of relationships were a great support for our vaccine development efforts. We tried three main approaches over the first ten years:
- A collaboration with Thailand’s Mahidol University aimed at developing classically and empirically attenuated vaccines specific to each serotype.
- A collaboration with the US Centers for Disease Control and Prevention to develop a tetravalent vaccine using an attenuated D2 vaccine virus that could serve as a “backbone” for expressing the structural antigens from D1, D3 and D4.
- A collaboration with Acambis (Cambridge, England, and Cambridge, USA) to use the YF17D vaccine strain of the yellow fever virus as a backbone to encode D1–D4 structural antigens.
In the early phases, we took all three of these approaches forward in parallel, but in the mid 2000s we decided to focus on the Acambis approach, because it had the best preclinical and manufacturing profile. The YF17D vaccine strain has been used for decades to induce protection against the yellow fever virus, so it’s well characterized. In fact, a key factor for the success of Dengvaxia was the ability of the 17D backbone to provide a very controlled, consistent attenuation profile across all four dengue serotypes, so that we could be confident of producing the vaccine at industrial scale. Dengvaxia is actually a combination of four different vaccines, each comprising a 17D backbone in which the yellow fever pre-membrane and envelope genes have been replaced with the corresponding genes encoding the structural antigens from either D1, D2, D3 or D4.
Marie-José: To emphasize Bruno’s point about teamwork, I have to say that Dengvaxia’s development would not have been possible if Sanofi Pasteur had not convened a large network of technical, medical and scientific collaborators, as well as the co-operation of the communities in the endemic countries that participated in the clinical studies. For a challenge like this, no single scientific body could have all the expertise that was needed. The challenge of this vaccine development was firstly to get the attenuation right, meaning the right balance between immunogenicity and safety, and we had to do that four times, once for each serotype. And it wasn’t straightforward, because dengue is an RNA virus, so it’s inherently unstable at the genetic level. Secondly, we had to mix the four attenuated constructs in a proportion such that they didn’t compete with each other for a protective immune response. In other words, we had to ensure that the formulation would consistently generate a balanced immune response against all four serotypes, without any one of them dominating. That’s why, as Bruno outlined, we adopted a parallel approach upfront, because at the start it wasn’t clear which strategy would be successful.
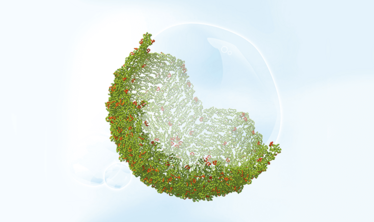
3D model of dengue virus capsid structure [credit: Sanofi Pasteur].
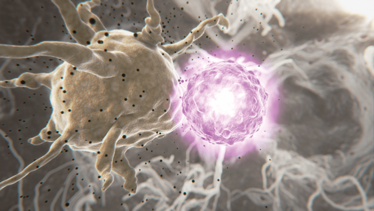
During the incubation period, the dengue virus replicates locally and then spreads into the bloodstream [credit: Sanofi Pasteur].
Did you ever doubt whether you could succeed?
Bruno: It was like a Snakes and Ladders game. Sometimes we got ladders and could move forward; sometimes we encountered a really big snake and we would then slide back down, and have to start over again but be wiser. Collaborating and having the support of a broad network gave us longer ladders - we gained more knowledge and more confidence in our strategy. But occasionally we would land on a snake, like when one of the first generation vaccines showed too much reactogenicity in humans and we had no choice but to abandon it. In a complex project like this, you don’t go from point A to point B in a direct line – that’s why it took 20 years to get to where we are now.
You’ve scaled up production very rapidly since the approval – how?
Marie-José: We had to anticipate success; we were determined to get the vaccine to at-risk populations in the shortest possible time scale, and so we built the manufacturing plant before we were certain of getting the vaccine approved. If we had waited to get the license before investing in manufacturing facilities, it would have been a long time before we could start vaccinating populations in need. You could say it was a risky strategy, but it was all part of our commitment to making dengue the next vaccine-preventable disease.
How did you feel when you heard that the vaccine had been approved?
Marie-José: It’s difficult to put into words. It was like a firework display of emotions!
Bruno: It was a feeling of satisfaction – but also of relief, because there’s always the worry that something could go wrong. That said, although it’s the end of the R&D phase, it’s just the start of the vaccine’s life in the real world.
You must be proud of your role in the Dengvaxia story…
Marie-José: It’s a tremendous achievement, but it is a collective achievement. I’m not sure if pride is the right word – there are so many emotions it’s difficult to pick one. We know that we’re on the eve of making a real impact in public health, and that our work will have a real impact on dengue and on people who suffer its effects. So it may be pride, it may be happiness, but, for sure, it is an honor and a privilege to have contributed.
Bruno: That’s right. Nobody can claim that they were “owner” of one part of the program or another – this entire project depended on teamwork. As well as the external collaborators I already mentioned, over one thousand Sanofi Pasteur employees have been involved, in one or another way, in the development of this vaccine.
What’s next for you both? Or rather, how can you follow something this big?
Marie-José: I always said that I didn’t want to leave the company prior to the vaccine reaching the market. I really wanted to see it through to approval, and maybe move on after that. But, as a matter of fact, now that it’s got to market, I’m still with Sanofi, although I’ve left R&D to work more closely with our CEO.
Bruno: I’m still 100 percent involved in dengue. As we said before, having the license is not the end – the most critical part of the Dengvaxia story starts now. Research still has an important role in supporting the new vaccine’s implementation in real-life situations; for example, in terms of interactions with other viruses, including for instance the emerging Zika virus. There is still much to be understood. If dengue vaccine development is like climbing Everest, then we are now at the final camp IV, just before the summit. The roll-out of the vaccine should take us to the top.
- Guy et al., Development of the Sanofi Pasteur tetravalent dengue vaccine: One more step forward. Vaccine 33, 7100–7111 (2015).
- RS Hadinegoro et al., Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med. 373, 1195–1206 (2015).
Associate Director, Dengue Scientific Affairs.
Formerly Vice-President Product Conception & Development.