
Arresting ALS
When it comes to prognosis, the mouse models I use to study amyotrophic lateral sclerosis (ALS) are sadly accurate – like their human counterparts with familial ALS, all mice with these mutations die young. After 20 years of trying – and failing – to find a treatment that could extend life, I was close to giving up, until a new drug candidate came along. Then something amazing happened – a mouse lived.
Every day I receive emails from ALS patients and their loved ones, which is both gratifying and heart breaking. The time from onset of the disease to death can be just a few years, and patients are desperate for some hope. The possibility that my work might help these people is a large part of what has kept me going for over 20 years, despite all the setbacks and frustrations.
My ALS journey actually started with an interest in oxidative stress. I was studying the oxidant peroxynitrite, which mediates tyrosine nitration – a process you can find in stroke, diabetes, heart disease, neurodegenerative disorders and many other conditions. One of the major antioxidant defenses that prevents the formation of peroxynitrite and protects the body from oxidative stress is the protein superoxide dismutase (SOD). But SOD also has a dark side – we discovered that it can catalyze tyrosine nitration, speeding up oxidative damage.
In 1993, it was found that some inherited cases of ALS are caused by mutations in the SOD-producing gene SOD1 (1). Our research group hypothesized that in these patients, SOD would catalyze tyrosine nitration and make the disease worse – a toxic gain-of-function. Soon after, we discovered that mutant SOD did exactly that (2), and ever since we’ve been focused on better understanding the role of SOD in ALS, and ultimately finding a therapy.
Developing mouse nerve cells. Credit: Torsten Wittmann, University of California, San Francisco
A cloud with a copper lining?
In many diseases – even intractable enigmas like Alzheimer’s – therapies work in mouse models, but cannot be translated into humans. In ALS – a disease whose pathology has been cited since 1824 and named since 1874 (3) – there had never even been a single functionally effective therapy in a mouse model. To this day, only one drug – riluzole – has been approved for use in the treatment of ALS, and that came onto the market 20 years ago, and increases life expectancy by only 2–3 months (4).
Over the years, we made many discoveries that kept pushing our knowledge a little bit further, but nothing that we could pursue as a possible therapy. The SOD1 mutant mice still died at around 130 days. Eventually, we decided to shift focus – if we couldn’t slow the disease process, could we speed it up? If we know how to break something, we reasoned, we might get a better idea of how to fix it.
Copper and zinc are essential for the maturation of SOD, and in ALS mouse models a lack of copper binding causes accumulation of mutant, immature SOD. A paper published in 2007 revealed that if you overexpress the copper chaperone for SOD (a metalloprotein named CCS) in wild-type mice, the mice are perfectly fine. But if you overexpress CCS in an ALS mouse model, the mice start dying six or seven times faster (6). That was an interesting discovery, because all human ALS patients have comparatively high CCS relative to SOD.
A former student of mine – Blaine Roberts – visited our laboratory and talked about a compound being studied by the Florey Institute in Melbourne (where he is now head of metalloproteomics), called copper-ATSM (CuATSM). The compound is traditionally used in PET imaging but Peter Crouch’s lab, alongside Roberts, had shown that it can improve locomotor function in ALS model mice (7). CuATSM delivers copper to the brain, and we suspected that it would counteract the copper deficiency seen in ALS mouse models.
A breakthrough
We acquired mice from the CCS study (6) – which developed ALS symptoms relatively slowly – and cross-bred them with the standard (SOD1-G93A) ALS mouse model, resulting in mice that died in 8–14 days. The first hurdle we faced in testing the drug with this model was how to get the CuATSM into tiny four or five day old mice, who were already runts. Our solution came when we found that CuATSM is soluble in dimethyl
sulfoxide (DMSO).
As we pipetted the CuATSM/DMSO solution onto the backs of the baby mice, it was absorbed through the skin within minutes, turning it bright red. Soon after, we watched the color fade as the solution was distributed into the subcutaneous fat, then subsequently to the brain and other organs via the bloodstream. Now the question became – would CuATSM affect disease progression?
Straight away, the mice started to improve markedly. They gained weight, and a few days later, far from being at death’s door, they showed no signs of disease. They began to develop quite normally, and to our surprise they passed the crucial 130-day mark, then 150 days, then 200. It was around day 230 when the first mouse became sick, but the others made it beyond their first birthday – unheard of in ALS research!
But our first response wasn’t elation, instead it was “something has to be wrong”. Our first thought was that the transgenes could have become inactivated, and only once we had ruled that out did we allow ourselves to get a little excited. Even then, we wanted to be really sure that the findings were airtight, so we immediately embarked on a series of very carefully controlled trials. It was difficult to bite our tongues, because we saw these results just as the ice bucket challenge was becoming a global phenomenon and everyone was talking about ALS (see "Putting ALS on Ice").
Putting ALS On Ice
By Joe Beckman
The ice bucket campaign accelerated the momentum of ALS research; plus, it educated people about the disease and the challenges faced by those living with it. We have been fortunate to receive ongoing funding from the Department of Defense that has kept our lab going through some tough times, and the ice bucket challenge has given that opportunity to other researchers through the extraordinary generosity of so many people around the globe.
After the campaign, it was great to see a lot of undergraduates coming to my lab looking to work on ALS, all of whom were incredibly motivated and passionate. My hope is that the attention and funding generated by the campaign is the beginning of growing interest, and not just a temporary blip on a radar.
What a difference a year makes
Fiscal year ending Jan 31st 2014 -
$8.4 million in contributions
Fiscal year ending Jan 31st 2015 -
$121.4 million in contributions
($115 million from the ice bucket challenge)
How the money will be spent
67% Research.
20% Patient & community services.
9% Public and professional education.
2% Fundraising.
2% Processing fees.
Data from the ALS Association.
We spent a lot of time considering sample sizes, consulting with statisticians about the setup and animal specialists about breeding the mice. We also blinded the study since the CuATSM/DMSO could stain the fur. We did that all to maximize the amount of good data we could obtain. Initially, we were worried about CuATSM toxicity but it soon became clear that there was no serious toxic effect on the mice, so we were able to increase the dose considerably. Ultimately, we settled on treating pups from the age of 5 days, with 30 mg/kg/dose of CuATSM twice daily, and the results surpassed all our expectations. We extended the lives of the standard SOD1 mutant ALS mouse model by 25 percent, and the ALS mouse model with both SOD1 mutation and overexpressed CCS by an amazing 500 percent (8). What’s more, cessation of CuATSM caused the mice to develop ALS symptoms, and restarting therapy rescued them.
ALS Genes
Genotypes of familial ALS and their associated chromosomes
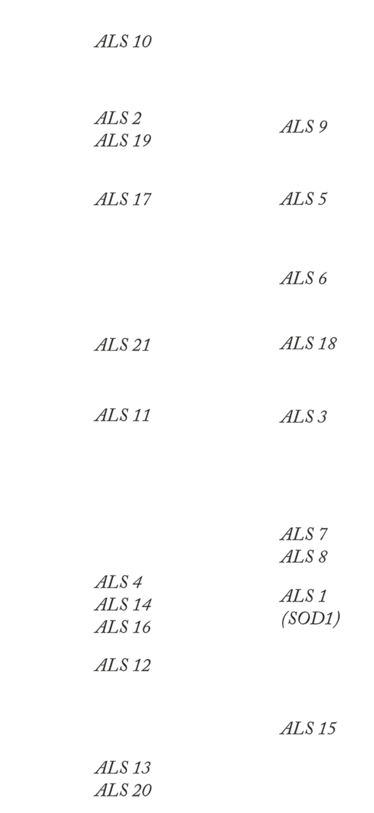
Trials and tribulations
We carried out a lot more experiments to reproduce certain elements of our findings, and compared our results with those of the drug’s developers in Melbourne, so by the time the paper came out we felt confident in our results. Despite this, we were met with skepticism from some of the peer reviewers, who were concerned that the mouse model may not translate into human patients. Only 2–7 percent of all ALS patients have a SOD1 mutation, and some consider this familial form a separate disease to sporadic ALS. Although it makes up the vast majority of cases, very little is known about the etiology of sporadic ALS – which is why most ALS researchers study familial forms. I believe there is a definite possibility that the drug might work in sporadic ALS patients – after all, the SOD1 mutation amplifies traits of the wildtype protein. But even if the drug does only work in the SOD1 mutation patients, it would be a much needed breakthrough for the disease.
The Melbourne group have taken the lead in developing the compound for Parkinson’s disease and ALS. Their license was granted to a newly formed company called Procypra, based in the USA, which is developing CuATSM for treatment of Parkinson’s and ALS.
Research Roundup
With a boost in funding and awareness from the ice bucket campaign, ALS research is moving fast. Here are just three of the latest advances.
Symptom relief
AB Science SA have recently announced the success of their phase 2/3 trials investigating the efficacy of a protein kinase inhibitor – designated masitinib – in improving the severity of disability according to the ALS functional rating scale (ALSFRS-R) of patients (9). The drug targets mast cells and macrophages through inhibition of certain kinases, and affects symptoms associated with CNS-related diseases, including ALS.
Role for retrovirus
Researchers from the NIH recently published a paper citing that human endogenous retrovirus-K (HERV-K) plays a role in sporadic ALS (10). The researchers found the virus expressed in cortical and spinal neurons in ALS patients, and discovered that HERV-K and its envelope proteins may contribute to neurodegeneration.
Misfolding mutants
A paper investigating the propagation of ALS lends weight to the idea that mutant SOD protein spreads via a prion-like mechanism. Mice which had a mutation in the SOD1 gene but had not yet developed symptoms, were injected with material from the spinal cords of affected mice, containing misfolded, mutant SOD proteins. Mice who received the injection developed ALS symptoms within months, which spread in a pattern similar to patients with ALS (11).
Where to next?
The identification of new genes is driving the field forward – we need to find out what is making things go wrong before we can start to think about how to fix it. There are now over 20 different types of genetic mutations linked with ALS, each one opening up new avenues of research (see "ALS Genes" and "Research Roundup"). However, it’s unfortunate that the SOD1 gene has been neglected by researchers who feel that, after decades of study with very little progress, it’s a dead end. I’m hopeful that our results will change that perception and encourage other researchers to start using this model again.
Right now, the team and I are working really hard on finding other variants of the CuATSM drug that will deliver enhanced results; the drug that we have right now works much better than the first 20 versions we tried, so there’s definitely hope that we can keep improving. We’re also advancing the mass spectrometry methods that we use to measure what’s happening inside the motor neurons, to give us an even clearer view into the mechanisms of disease. Thanks to those efforts, we already have a pretty good idea about how SOD causes motor neurons to die, but we want to keep honing those ideas until we can address all the potential criticisms.
I’m really excited about the field right now. The ice bucket challenge put ALS in the spotlight and injected much-needed funding. Now we must continue to drive research forward until we have an effective treatment in the clinic. We understand so much more about the mechanisms of the disease, that it no longer seems like a hopeless task. Research has reduced the disease to something we can attack, and hopefully defeat.
Joe Beckman is the Principle Investigator, and Burgess and Elizabeth Jamieson Chair, in Healthspan Research at the Linus Pauling Institute, Oregon State University, OR, USA.
- DR Rosen et al., “Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis”, Nature, 362, 59-62 (1993). PMID: 8446170.
- JP Crow et al., “Decreased zinc affinity of amyotrophic lateral sclerosis-associated superoxide dismutase mutants leads to enhanced catalysis of tyrosine nitration by peroxynitrite”, J Neurochem, 69, 1936-1944 (1997). PMID: 9349538.
- LP Rowland, “How amyotrophic lateral sclerosis got its name: the clinical-pathological genius of Jean-Martin Charcot”, Arch Neurol, 58, 512-5115 (2001). PMID: 11255459.
- RG Miller et al., “Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND)”, Cochrane Database Syst Rev, 14, (2012). PMID: 22419278.
- Prize4life, “Treatment Prize”, (2014). Available at: bit.ly/1qtJHgn. Accessed April 6, 2016.
- M Son et al., “Overexpression of CCS in G93A-SOD1 mice lead to accelerated neurological deficits with severe mitochondrial pathology”, Proc Natl Acad Sci USA, 104, 6072-6077 (2007). PMID: 17389365.
- BR Roberts et al., “Oral treatment with CuII(atsm) increases mutant SOD1 in vivo but protects motor neurons and improves the phenotype of a transgenic mouse model of amyotrophic lateral sclerosis”, Neurobiol Dis, 34, 8021-8031 (2014). PMID: 24899723.
- JR Williams et al., “Copper delivery to CNS by CuATSM effectively treats motor neuron disease in SODG93A mice co-expressing the Copper-Chaperone-for-SOD”, Neurobiol Dis, 89, 1-9 (2016). PMID: 26826269.
- AB Science, “Masitinib in amyotrophic lateral sclerosis (ALS)”, (2016). Available at: bit.ly/1N8mef1. Accessed April 13, 2016.
- W Li et al., “Human endogenous retrovirus-K contributes to motor neuron disease”, Sci Transl Med, 7, 307a153 (2015). PMID: 26424568.
- JI Ayers et al., “Prion-like propagation of mutant SOD1 misfolding and motor neuron disease spread along neuroanatomical pathways”, Acta Neuropathol, 131, 103-114 (2016). PMID: 26650262.
Joe Beckman is the Principle Investigator, and Burgess and Elizabeth Jamieson Chair, in Healthspan Research at the Linus Pauling Institute, Oregon State University.















