
An Islet Odyssey
With regular insulin injections, Type 1 diabetes is a manageable condition for most. But what happens when it becomes too difficult to keep blood sugar under control? Cell therapies being developed on both sides of the Atlantic could offer hope to patients running out of options – and might even hold the key to a cure.
Keith Thompson can vividly remember the first patient treated at Scotland’s islet transplantation unit. Then head of the Scottish National Blood Transfusion Service, Thompson helped set up the program with Edinburgh surgeon John Casey in 2011. The patient had severe hypoglycemia unawareness and, unable to tell when her blood sugar was dangerously low, she suffered frequent and sudden blackouts.
“Some of these patients have to be woken up at intervals through the night because they are in danger of going into a coma. They are reluctant to go out for fear of collapsing in the street. It’s just dreadful. And yet within a few weeks of her islet transplant that first patient was off insulin and living a normal life. It was wonderful to see,” says Thompson. “People often use the term ‘transformational’ to describe a new drug or treatment, but until you’ve seen it for yourself, it’s hard to imagine the difference it can make.”
Transforming lives
Soon, the Scottish unit was carrying out about 15 transplants each year, bringing the UK total up to 50–80 per year. However, they represent only a fraction of the 14,000 patients in the UK who suffer with frequent, severe hypoglycemic events; as with most transplants, the limiting factor is the availability of donor organs.
Soon after that first Scottish islet transplant, Thompson took on a new role as CEO of the UK’s Cell and Gene Therapy Catapult (www.ct.catapult.org.uk), but the life-changing nature of the procedure left a strong impression. Two years ago, he had a visit from Casey and his longtime collaborator, Aberdeen University researcher Kevin Docherty. Docherty’s research focuses on the biology of islets, with a particular emphasis on the transcription factors that control islet development. Previously, he had worked on reprogramming embryonic stem cells into insulin-producing cells. Now, he had applied his knowledge to reprogram non-insulin-producing (exocrine) pancreatic tissue from donor organs into functional islets. By transforming the leftover tissue from a donor pancreas after islet extraction into more islets, the procedure could provide 10 to 100 times more islets from a single donor.
Thompson was immediately grabbed by the idea. “I was so impressed by the difference that islet transplants made to patients’ lives, that the opportunity to offer the procedure on a larger scale was rather like the ‘monoclonal moment’ for me,” says Thompson, “When I saw monoclonal antibodies for the first time, I was immediately convinced that they could transform the world, and I feel the same about cell therapies.”
After a few amendments to the protocol to further increase the scale-up of the transformed cells, and some successful pre-clinical studies, the Catapult signed a research collaboration agreement to help accelerate the research. And earlier this year, a new company – Islexa – was formed by the Catapult in partnership with the University of Aberdeen and their collaborators at Scotland’s National Blood Transfusion Service, Edinburgh University and NHS Lothian.
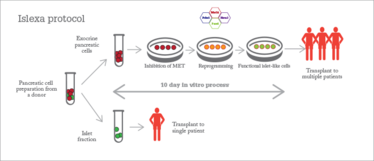
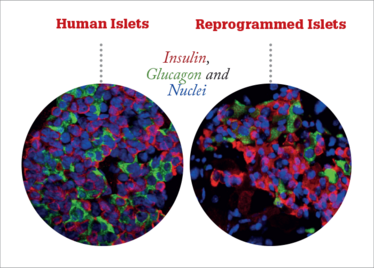
Different approach, same goal
Islexa won’t be the first company to set its sights on lab-grown alternatives to islet transplantation. US company Viacyte (www.viacyte.com) are already carrying out clinical trials of their human embryonic stem cell (hESC)-derived insulin-producing cells. Encapsulated in a delivery device that protects the cells from the immune system, the cells proved safe and effective when inserted under the skin in mouse models, and many experts are tipping it to be the first breakthrough hESC-derived product – the ongoing clinical trial is even the subject of a documentary film (www.thehumantrial.com).
Thompson is enthusiastic about the work coming from Viacyte and others, “We think they’re doing a great job, and anything that can push forward this technology is great.” But the team believes that the technology being developed by Islexa could add another approach – after all, the more shots taken, the greater the chance of scoring a goal.
One advantage of the Islexa approach is that the exocrine pancreatic cells are transdifferentiated to a mesenchymal intermediate stage, then differentiated into endocrine cells – they are never pluripotent. Pluripotent cells have the potential to cause cancer, so cell therapies derived from them have to be very carefully checked to make sure all the cells are fully differentiated.
At just 10 days, the total cycle is faster than creating beta cells from a pluripotent cell line, and the resulting islets are very similar to naturally occurring human islets. They are a similar size and shape, and contain a normal ratio of different cell types. As well as insulin-producing beta cells, the islets contain alpha and delta cells, which produce the blood sugar-regulating hormones glucagon and somatostatin. “The end result is functional islets that, for all intents and purposes, behave like human islets,” says Thompson.
The resulting islets had an “immediate and prolonged effect in normalizing blood glucose levels” when transplanted into diabetic mice (1), and the team predict that a transplant of around 3 billion cells into a human patient should have an immediate therapeutic effect.
A straight swap
There is more pre-clinical safety work to do before the first patients are treated, but Thompson hopes to see the first clinical trials start in 2019. The researchers are hoping for a relatively smooth road to translation: “So many new products or drugs require completely new clinical pathways, but our islets can fit into the current pathway for islet transplants,” says Thompson. Indeed, a long career in the biotech industry has taught Thompson that the fastest route for new technologies to gain a foothold in the clinic is through simple substitution. Examples from biotech include recombinant insulin replacing porcine insulin for diabetes treatment, and recombinant factor VIII blood clotting factors replacing plasma-derived versions. In both cases, the synthetic product not only replaced the original, but significantly expanded the availability of the treatment.
That current standard is the Edmonton protocol, developed in the late 1990s by James Shapiro and colleagues at the University of Alberta, Edmonton. The protocol was the first to offer consistent success rates for islet transplantation and is still in use today. Donor islets are infused through the patient’s portal vein to lodge in the liver and are protected from the body’s immune system by a mild immunosuppressive regime. It’s hoped that the lab-grown islets can be administered in exactly the same way, as a direct swap for donor islets. “The only variable is the source of the islets,” says Thompson.
For now, the focus is on patients who would be considered for a donor islet transplant – those with serious hypoglycemic episodes and unawareness, in whom the risks of immunosuppression are outweighed by the benefits of better glucose control. In the long term, there could be the potential to avoid immunosuppression, for example by encapsulating the cells, which could open up the treatment to less severely affected patients and those with contraindications for immunosuppression. “Many patients maintain a good quality of life with regular insulin injections, so right now we’re really focused on those patients who have the greatest need. But if over time the treatment proves safe and cost effective, then there could be potential to take it further,” says Thompson.
If clinical trials are successful, Islexa should certainly have no trouble attracting investment, suggests Thompson, “Worldwide the total number of patients with diabetes is estimated at 347 million, with 21 million of those suffering from Type 1 diabetes. Most Type 1 diabetic patients will have some hypoglycemic episodes, but about 10 percent of them will develop hypoglycemia unawareness, and about five percent will experience more than seven serious hypoglycemic attacks each year. That is a major unmet need.”
Hidden Danger
The high price of hypoglycemia unawareness
Usually, the body responds to abnormally low blood sugar by releasing stress hormone epinephrine (adrenaline), which causes sweating, shaking and weakness. For a diabetic, these are important warning signs of a “hypo” (hypoglycemia), and prompt the patient to raise their blood sugar with a snack or soft drink.
Over time, particularly in patients who swing from very low to very high blood sugar, the epinephrine response can become blunted or disappear altogether. With no early warning signs, these patients may only become aware of a hypoglycemic episode once their blood sugar dips so low that they become confused, disorientated, or unconscious.
Hypoglycemia unawareness can be very dangerous – a severe hypoglycemic episode can result in seizures, brain damage, or even death. Some patients are forced to rely on continuous monitoring devices or specially trained service dogs to alert them to falling sugar levels
The Cell and Gene Therapy Catapult
Accelerating clinical translation in the UK’s gene and cell therapy industry.

The UK has a global reputation for innovative basic science and technology, but hasn’t always been successful when it comes to translating new advances to a commercial scale.
In a bid to close the gap, the Catapult program was born. There are now 11 Catapult centers around the UK, covering everything from renewable energy to precision medicine. Each Catapult is set up as an independent not-for-profit company, funded in three ways: a core grant that funds key infrastructure and projects, collaborative R&D grants for specific projects with business, and contract research on behalf of companies.
The Cell and Gene Therapy Catapult received a core grant of £70 million for the first five years, which paid for state-of-the-art laboratory facilities and a team of 120 people – all specialists in cell and gene therapy. The key focus is identifying and overcoming barriers to growth in the industry, including:
- Reimbursement. The health-economics and market access for these types of therapies is poorly understood, which makes it hard for companies to make accurate financial predictions. The Catapult set up a health economics and market access unit to carry out research in this area and act as a consultancy for companies.
- Technical assistance. As an emerging industry, there are a number of technical hurdles for cell and gene therapies. Scale-up, delivery, and GMP are all areas that university spin-outs and other small companies may have limited expertise in. The Catapult is dedicated to finding the best ways to move cell and gene therapy products into commercial production.
- Pre-clinical and clinical trials. The Catapult has a team of regulatory specialists, working closely with UK, European, and other regulators, all of whom are actively looking for guidance and help in this industry. The Catapult also has experience and capability in clinical trial development and delivery for cell and gene therapies.
- Manufacturing. There is an abundance of small GMP cell manufacturing sites in the UK – mainly located within universities – that can be used for early-stage products for trials. However, there is a lack of large-scale capacity. Companies have been reluctant to invest in manufacturing facilities themselves until their products are further along the pipeline, while CMOs have been hesitant to increase building capacity to service an emerging industry. To fill the gap, the Catapult raised another £55 million from the UK government to build a world-leading, large-scale (7200 square meter) manufacturing center. Due to open next year, the center will allow multiple clients to to manufacture products under GMP conditions in order to carry out pivotal trials.
Though the raison d’être of the Catapult is to promote the cell and gene therapy industry in the UK, the team works with collaborators all around the world, from Europe to Japan to the USA. And it seems other regions are taking note – the California Institute for Regenerative Medicine (CIRM) recently announced the launch of a new Accelerating Center, providing regulatory, clinical trial and data management services to smooth the path to the clinic for novel cell therapies.
Spin out translation
The Catapult also recognizes that certain innovative – but tentative – directions need an extra boost, and is able to spin out companies to forge new paths. “We are always on the lookout for exemplar projects that we think have the potential to move things forward, but are just a bit too early for industry to pick up. By spinning these projects out into companies, we’re not only helping get innovations to patients faster, but learning how to solve some of the bigger problems faced by the industry as whole,” says Keith Thompson, CEO of the UK’s Cell and Gene Therapy Catapult.
Islexa is the third company launched by the Cell and Gene Therapy Catapult. Catapult Therapy TCR Ltd – a collaboration with University College London and Imperial College London – was the first and, despite a somewhat prosaic name, is an exciting addition to the cancer immunotherapy boom. Its TCR gene-modified T cells (designed to target WT1-overexpressing cancer cells) were originally developed by the Leukemia and Lymphoma Research Society but, despite promising pre-clinical results, the project stalled prior to clinical trials. To date, five patients with acute myeloid leukemia and myelodysplastic syndrome have received the cell therapy, with no serious adverse events, and the trial is being expanded into several European centers.
The start of 2016 saw the advent of the second spin-out company, Chimeric Therapeutics Ltd, which was founded on CAR-T cell research carried out at the University of Birmingham. CAR-T immunotherapy has shown amazing results in hematological cancers. Chimeric hopes to translate that efficacy into solid tumors, by targeting angiogenesis marker CLEC14a, and is currently in pre-clinical development. For all Catapult companies, the aim is to move the technology to a stage where it is attractive to additional investors, so that they can eventually become independent of Catapult funding.
A Preventative Approach
Expanding the availability of islet transplantation could bring huge benefits to millions of diabetics worldwide. But what if we could prevent the destruction of islets in the first place?
Type 1 diabetes is caused by a breakdown in immune tolerance that results in immune cells attacking insulin-producing beta cells in the pancreas. Jeffrey Bluestone and colleagues at the University of California, San Francisco (UCSF), are exploring a different kind of cell therapy, which interrupts the autoimmune attack. Immunosuppressive agents have been shown to slow progression of Type 1 diabetes, but come with a host of side effects. Therapies that target T cells specifically can avoid some of the risks, but their efficacy is generally short-lived.
Previous research has suggested that defective regulatory T cell (Treg) responses play an important role in autoimmune disease. Tregs damp down induction and proliferation of various immune cells, including effector T cells, and so maintain tolerance to self-antigens. The UCSF researchers hypothesized that boosting the body’s supply of Tregs may be able to stop the immune system attacking insulin-producing beta cells, while maintaining a normal immune response to external pathogens.
Recently, the team reported results from a Phase I safety study in Type 1 diabetes patients. In the study, the patients’ own Tregs were extracted from blood samples and expanded in vitro, before being infused back into the blood. Fourteen patients with recent onset of Type 1 diabetes received infusions of between 5 million and 2.6 billion of their own Tregs, with no serious side effects. Around 25 percent of the polyclonal cells were still present after 90 days (2). An open-label study by a team of Polish researchers also used autologous Tregs to treat patients with Type 1 diabetes, and found some evidence of prolonged survival of islets (3).
The stage is set for further trials of Treg cells, and the California team is looking at the potential for a combination with drugs targeting effector T cells, or for genetically modifying the Tregs to specifically down-regulate immune responses against beta cells.
- MJ Lima et al., “Generation of functional beta-like cells from human exocrine pancreas”, PLoS One, 11, e015620 (2016). PMID: 27243814.
- JA Bluestone et al., “Type 1 diabetes immunotherapy using polyclonal regulatory T cells”, Sci Transl Med, 7, 315ra189 (2015). PMID: 26606968.
- N Marek-Trzonkowska et al., “Therapy of Type 1 diabetes with CD4(+)CD25(high)CD127-regulatory T cells prolongs survival of pancreatic islets - results of one year follow-up”, Clin Immunol, 153, 23 (2014). PMID: 24704576.

“As Editor of The Translational Scientist, I’m working closely with our audience to create vibrant, engaging content that reflects the hard work and passion that goes into bringing new medicines to market. I got my start in biomedical publishing as a commissioning editor for healthcare journals and have spent my career covering everything from early-stage research to clinical medicine, so I know my way around. And I can’t think of a more interesting, challenging or important area to be working in.”















