Tackling Old Age Before Birth
Understanding the factors that influence epigenetic changes throughout life offers us the potential to combat diseases like osteoporosis – before they manifest.
At a Glance:
- Osteoporosis is a major culprit for fractures in the elderly, comes with a huge economic cost, and has no cure – but what if it could be prevented?
- By understanding the factors that influence health – from pre-conception into adulthood – we have the opportunity to change the course of human disease
- Studying the epigenome can provide a molecular record of life events and teach us what interventions might improve bone health
- Our studies indicate that vitamin D supplementation during pregnancy could have an effect comparable to anti-osteoporotic medications in preventing fractures in later life, if the differences observed at birth persist into older age.
Osteoporosis is a skeletal disorder characterized by low bone mass and loss of the normal bone microarchitecture, which leads to increased bone fragility; it causes more than 8.9 million bone fractures worldwide every year. Data suggests that, in the UK, one in two women and one in five men aged 50 are expected to have an osteoporosis-related fracture in their remaining lifetime (1). Clearly, this has a huge economic cost, and comes with considerable mortality and morbidity – hip fractures are associated with an increased mortality rate of 10–20 percent in the first year after fracture, and a significant number of people will require long term residential or nursing care. There is no cure, although the condition can be managed through lifestyle changes and medications.
But what if you could protect people from osteoporosis before they’ve even been born?
Looking at the bigger picture
At the Medical Research Council (MRC) Lifecourse Epidemiology Unit, University of Southampton, we work towards improving human health at a population level, by understanding factors which influence human health and development from pre-conception, through pregnancy, and then childhood. The idea that epigenetic modifications may influence later bone health stems from the fetal programming hypothesis. It proposes a developmental model for the origins of disease, where maternal, infant and childhood nutrition, health, exposure to infections, and lifestyle permanently “program” an individual’s metabolism and growth – and, in doing so, determine the pathologies of old age.
The premise of developmental plasticity (that a single genotype, influenced by specific intrauterine events, has the capability to produce different phenotypes) is now widely accepted. Specific developmental periods are known to exist when an organism is sensitive, or plastic to, its environment. Every organism aims to develop a phenotype that is best suited to its environment. For example, a malnourished fetus will alter the structure and function of various organs – including the cardiovascular, endocrine and musculoskeletal system – to preserve neurodevelopment, and to enable its survival in the womb.
Making connections
The first clues that the early life environment may be important in determining the risk of diseases as an adult came from studies of coronary heart disease. It was noted that the incidence of coronary heart disease in Britain was twice as high in poorer areas of the country, and in lower income groups. Studies of the rates of death from coronary heart disease throughout England and Wales were shown to parallel death rates in babies (2), and analyses of birth registries in the county of Hertfordshire, UK, were the first to show that birth weight at the lower end of the normal range was associated with higher rates of coronary heart disease and type II diabetes in later life. These findings have since been extensively replicated, in both men and women in Europe, the USA, China and India, and low birth weight was also shown to be associated with osteoporosis.
Studies from the MRC Lifecourse Epidemiology Unit, directed by Cyrus Cooper, and from elsewhere, have shown that maternal lifestyle, body build and vitamin D status during pregnancy influence childhood bone health, and so do factors in childhood including postnatal nutrition, body composition and physical activity. In addition, poor early growth in childhood has been shown to be associated with increased risks of hip fracture in old age.
But what can we do with this information? As osteoporosis becomes an increasingly pressing public health issue with the aging population, more research is being directed towards ways of preventing osteoporotic fracture (3) to understand the factors affecting the peak bone mass (PBM) attained by individuals during growth and development, and to learn which factors affect the rate of subsequent bone loss. PBM is defined as the maximum total skeletal mass accrued at the completion of skeletal development, usually in the third or early fourth decade of life, and is a key component of the mechanical strength of bone. It has been shown in mathematical modeling studies to be a more powerful predictor of the age of osteoporosis development than age at menopause or rate of subsequent age-related bone loss – for example, a 10 percent increase in PBM delays the onset of osteoporosis by 13 years. Acquiring optimal peak bone mass is therefore essential to bone health as an adult, so targeting the developmental processes that influence bone mineral accrual during early life may be the key to future prevention of the disease, and is central to our line of research (1)(3).
Famine, bone, and epigenetics
How is optimal PBM achieved? It is, of course, governed in part by genetic factors – but there is increasing evidence that some residual variance in both PBM and future fracture risk may be explained by the environment’s influence on gene expression, both in utero and in early life (see Figure 1). Such changes can be caused by epigenetic mechanisms – where gene expression is modified without changes to the DNA code itself. Epigenetic signals are essential in determining when and where genes are expressed, and the epigenome can be considered as a molecular record of events, accumulating throughout a lifetime. For example, monozygotic twins have been shown to be most similar from an epigenetic point of view at birth, with their epigenomes diverging at a slower rate if they share a common environment.
Epigenetic mechanisms include DNA methylation, histone modification and non-coding RNA, but DNA methylation is the most widely studied. Studies of the Dutch Hunger Winter of 1944–45, a famine which occurred following the German occupation of the Netherlands, provide evidence that maternal nutrition influences offspring health in later life and suggest that the timing of the nutritional restriction is important (reviewed in 4), with women exposed to the famine during mid-to-late gestation having babies with significantly reduced birth weights. Babies whose mothers were exposed only during early gestation had normal birth weights; however, they grew up to have higher rates of obesity and cardiovascular disease than those born before and after the war, and higher rates than those exposed during mid-to-late gestation. Comparable findings are now well established in a variety of animal models where nutrition can be precisely controlled, and alterations in the methylation of a number of genes have been found, with the timing of the nutritional constraint appearing to be important.
We wanted to assess whether DNA methylation could be associated with bone health in a mother-offspring cohort, with the aim of identifying whether specific factors in pregnancy or of the maternal diet could be of importance.
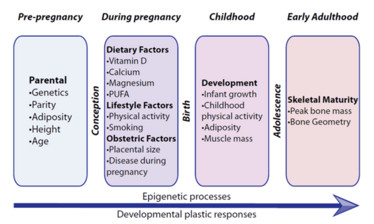
Figure 1. A summary of influences on early bone development. The magnitude of the variation in population risk of poor bone health increases with age. Conversely, developmental plasticity decreases markedly after intrauterine and early postnatal life. PUFA: polyunsaturated fatty acids.
Tracking bone health
At the University of Southampton, through collaboration between scientists at the MRC Lifecourse Epidemiology Unit and the Institute of Developmental Sciences, we have a well-established pipeline for the identification of epigenetic markers of various aspects of child health, including obesity and child bone health. But to conduct this kind of cohort work, a specialist infrastructure and a large team of motivated people are needed – not just the scientists, but research nurses, radiographers, and administrative and laboratory staff too. Considerable organization is needed behind the scenes to arrange appointments, catalogue samples and log questionnaire data. It is also extremely helpful to work in a team where epidemiology can run alongside basic science: in our case, it has been of great benefit to be allied with a team of epigenetics specialists (run by Karen Lillycrop).
To assess DNA methylation, our group (led by Cyrus Cooper and Nicholas Harvey, and by Karen Lillycrop’s group at the Institute of Developmental Sciences, University of Southampton) performed methylation arrays using umbilical cord tissue samples from children who were carefully phenotyped, looking for associations between DNA methylation and particular traits. In the context of bone health, we have identified various differentially methylated regions of interest – including the CDKN2A gene (known to be important in cell cycle regulation) and RXRA (involved in vitamin D signaling, amongst other functions). We were then able to use pyrosequencing to look at the methylation of DNA at the individual base (CpG site) level, and understand more about the associations between methylation and offspring phenotype.
Because we’re looking at associations between DNA methylation and bone health in children, it is important for us to determine whether a measurement in early life (for example, bone mineral density) can be used to predict a future measurement in an individual. Tracking refers to the maintenance of a relative rank of an individual within a group, when a measurement is repeated at two or more time intervals. Therefore, evidence for high levels of tracking of the measurement or characteristic would support a higher likelihood that an early life intervention to improve the measurement would have a sustained positive effect over time.
The tracking of bone mineral density (BMD) and bone mineral content (BMC) has been well described in populations of different ages and ethnicities, supporting the notion that interventions to increase BMC could have sustained effects into adulthood.
We have shown that CDKN2A methylation is negatively associated with bone health (BMC, BMD and bone area) in children from the Southampton Women’s Survey (SWS), a well characterized mother-offspring cohort, at the ages of four and six years (as shown in Figure 2). A similar finding was published by Nicholas Harvey, who found that RXRA methylation is negatively associated with bone health in the same group of children, and replicated this finding in another cohort. There is some evidence that this process might be mediated by vitamin D, as increased vitamin D status has been associated with lower levels of RXRA methylation (5). We are now in the process of evaluating the effects of vitamin D supplementation in pregnancy on RXRA methylation in a randomized controlled trial – MAVIDOS (6).
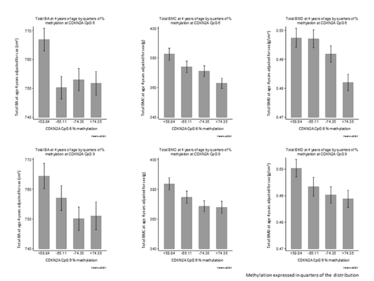
Figure 2. CDKN2A CpG methylation in relation to bone mineral outcomes. Percentage methylation at CDKN2A CpG 6 (top) and CpG 9 (bottom) expressed in quarters of the distribution in umbilical cord tissue, and offspring total bone area (whole body minus head, cm2), bone mineral content (g), and areal bone mineral density (g/cm2) at age 4 years. Reproduced with permission from EM Curtis et al (8).
Quantifying exactly how large an effect a change in methylation of CDKN2A has on fracture risk is challenging, but we are working towards a better understanding. Previous studies have tried to associate birth with fracture risk; in a meta-analysis, each 1 kg increase in birth weight has been shown to be associated with a 1.49 g increase in BMC at the lumbar spine and 1.41 g at the hip (95% CI 0.91, 1.91 g) in adulthood, with the effect independent of adult weight and BMI (7). The 1.49 g increase in adult lumbar spine BMC per 1 kg increase in birth weight is equivalent to a 0.13 Standard Deviations/kg change. If such differences tracked into adulthood, this could lead to a 21 percent reduction in risk of any fracture, or a 35 percent reduction in risk of osteoporotic fracture per 1 kg increase in birth weight.
In the MAVIDOS study, supplementation was found to improve bone health in babies born in winter months. For example, for winter births, the difference in whole body BMC between treatment and placebo offspring was approximately 0.5 SD, which, if maintained into adult life, would equate to a 50 percent difference in fracture risk – an effect comparable with many anti-osteoporotic medications (6). We are currently investigating whether epigenetic mechanisms (including methylation at RXRA and CDKN2A) could underlie this observed effect. In the field of epigenetics, it is particularly important to integrate associations observed between methylation at a particular locus and a health outcome with functional studies. It is vital to try to understand the mechanisms behind the observations at a molecular level, as this helps us in the future to integrate observation, mechanisms and future interventions to improve population health.
The implications of this particular study are that a cheap and simple intervention – such as vitamin D supplementation – could potentially be used on a population level to increase peak bone mass obtained, and therefore reduce skeletal problems in old age. By better understanding the things that influence us during our growth and development, we have the power to change them – in this case, a small effect on millions of people could go on to prevent thousands of bone fractures.
- N Harvey et al., “Osteoporosis: a lifecourse approach”, J Bone Miner Res, 29, 1917–1925 (2014). PMID: 24861883.
- DJ Barker, C Osmond, “Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales”, Lancet, 1, 1077–1081 (1986). PMID: 2871345.
- C Cooper, LJ Melton 3rd, “Epidemiology of osteoporosis”, Trends Endocrinol Metab, 3, 224–229 (1992). PMID: 18407104.
- LC Schulz, “The Dutch Hunger Winter and the developmental origins of health and disease”, Proc Natl Acad Sci, 107, 16757–16757 (2010). PMID: 20855592.
- NC Harvey, “Childhood bone mineral content is associated with methylation status of the RXRA promoter at birth”, J Bone Miner Res, 29, 600–607 (2014). PMID: 23907847.
- C Cooper et al., “Maternal gestational vitamin D supplementation and offspring bone health (MAVIDOS): a multicentre, double-blind, randomised placebo-controlled trial”, Lancet Diabetes Endocrinol, 4, 393–402 (2016). PMID: 26944421.
- J Baird et al., “Does birthweight predict bone mass in adulthood? A systematic review and meta-analysis”, Osteoporos Int, 22, 1323–1334 (2011). PMID: 20683711.
- EM Curtis et al., “Perinatal DNA methylation at CDKN2A is associated with offspring bone mass: findings from the Southampton Women's Survey”, J Bone Miner Res, [Epub ahead of print] (2017). PMID: 28419547.
Beth Curtis is a Clinical Research Fellow based at the MRC Lifecourse Epidemiology Unit, University of Southampton, UK. She is working towards a better understanding of the epigenetic determinants of bone health using the Southampton Women’s Survey mother-offspring cohort and the MAVIDOS randomized controlled trial of vitamin D supplementation in pregnancy.















