Oncotherapy Goes Viral
Robert Coffin tells the bench-to-bedside story of how the first FDA-approved oncolytic virotherapy, talimogene laherparepvec (T-VEC), was translated from a colleague’s cold sore to a potential global game changer.
How did it all begin?
In the 90s, I headed up an academic research group at University College London. We were studying herpes simplex virus (HSV), and we’d developed some HSV-based gene delivery vectors for gene therapy of nervous system disorders. We needed a commercial vehicle to fully exploit the potential of this technology, so in 1999 we set up Neurovex Ltd with a £3M investment from Merlin Ventures. Our initial focus was to develop gene therapies for neural disorders, such as Parkinson’s disease, chronic pain, and spinal injury. In parallel, however, we were applying the technology to cancer vaccine approaches. Initially, we took an ex vivo dendritic cell-based vaccine approach, but around 2001 we changed our focus to oncolytic virotherapy. We noticed that the oncolytic viruses being developed by other groups, while well-tolerated in patients, were just not potent enough to have a significant anticancer effect. We thought that we could improve on these by combining the oncolytic virus and tumor vaccine approaches. As Neurovex was no longer the most appropriate name for the company, it was renamed Biovex.
Did the death of a patient in an (unrelated) gene therapy trial in the late 90s dent your confidence?
We certainly saw a lot of negativity from investors and the media as a result of that event. But frankly, those of us who were actually working in the field could see that that the issue was not with the viral vector itself, but with how it was used. So although the death was tragic, we firmly believed it had no significant implications for the Biovex drug development program. But it’s also true that many investors did not apply any kind of logical or even rational analysis to the situation and for a while it became very difficult to get funding for anything that had a hint of gene therapy, cancer vaccines or even immunotherapy. It’s only recently that cancer immunotherapy has been “re-sanitized” by the approval of the checkpoint inhibitors.
At a Glance
International non-proprietary name (INN): Talimogene laherparepvec
Brand name: Imlygic
Previous name: OncoVEX GM-CSF
Developed by: Biovex (until 2011)
Marketed by: Amgen (acquired Biovex in 2011)
Drug class: Oncolytic immunotherapy (first in class)
Approval status: Approved by FDA (27/10/2015) for monotherapy of unresectable melanoma recurrent after initial surgery. Recommended for approval by EU committee (22/10/2015) for unresectable melanoma that is regionally or distantly metastatic, with no bone, brain, lung or other visceral disease.
Reference
- R. Andtbacka et al., J. Clin. Oncol. 33, 2780-2788 (2015).
Was T-VEC really derived from a colleague’s cold sore?
Yes – we isolated the HSV1 JS-1 strain by taking a swab from a cold sore that a colleague in London happened to have. We didn’t want to rely on laboratory strains of HSV, because they could have been affected by serial passage in cell culture. Lab strains can be attenuated in terms of their virulence in primary human cells, and may never have been particularly good at replicating in tumor tissue anyway, since that wasn’t what they had been selected for when they were first isolated. Sure enough, we found that all the clinical strains we tested were better at infecting human cells than the laboratory strains, and we picked the best one of these to be developed into T-VEC.
And how do you turn that into something that kills cancer cells?
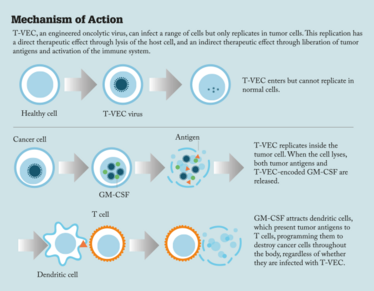
The normal antiviral response mechanisms of the cell – the interferon-based pathways – are closely interlinked with the tumor suppressor pathways. As a result, when tumor suppressor pathways are inactivated during the development of cancer, the antiviral response pathways tend to be disabled to a greater or lesser extent too. In consequence, the genes that viruses usually encode to evade the antiviral response are dispensable in tumors. If they are deleted the virus will no longer replicate in normal tissue, due to the host antiviral response, but will still replicate in tumors. We exploited this background by deleting the HSV gene encoding ICP34.5, which is involved in evading host antiviral responses, and in addition we deleted the ICP47 gene – a mutation which enhances viral replication selectively in tumors. Since HSV replication results in the death of the host cell and liberation of more viral particles, the resulting virus has an amplified lytic effect.
Directly injecting this virus into a tumor therefore causes lysis of the cancer cells as the virus replicates and spreads through the tumor. That’s important, but it’s only a local mechanism of action. We also wanted to enhance the systemic immune response to tumor antigens released through this process, in other words generate a patient-specific anti-tumor vaccine in situ within the patient. To achieve that we inserted the GM-CSF gene into the JS-1 vector backbone. GM-CSF attracts and activates dendritic cells – specialized cells of the immune system – which present antigen to T cells and thereby kick off a systemic T cell response to the tumor antigens. It has now become clear that the immune response generated in response to tumor neoantigens present within the tumor, but not present in normal tissue and therefore not recognized as “self”, is particularly important in achieving therapeutic benefit. By lysing tumor cells and thereby liberating these tumor antigens – and at the same time attracting and activating dendritic cells to signpost those tumor antigens to the immune system – we get not only a local tumor-killing effect, but also a systemic immune effect that clears distant metastases – including micrometastases – vaccinates against relapse, and improves overall survival.
Are there potential indications beyond melanoma?
Actually, the T-VEC Phase I trial was a standard late-stage solid tumor trial, enrolling not just patients with melanoma but also head and neck cancer and breast cancer patients. Biovex then conducted follow-on studies in head and neck cancer, melanoma and pancreatic cancer, and ultimately progressed T-VEC into Phase III in both head and neck cancer and melanoma. When Amgen purchased Biovec, they decided to initially focus on melanoma, although they are now planning to resume trials in head and neck cancer too.
Given the novelty of the approach, were there any specific regulatory challenges?
We always found regulators to be very helpful and collaborative, both in the UK and the US. They applied the proper level of caution at each step, but the path had already been smoothed by others who had conducted clinical trials with first-generation oncolytic viruses before us. So the regulators were already experienced with the approach when we started, with substantial safety data already generated, and they’ve got more comfortable with the product class since. At first, however, the conditions for clinical trials were quite stringent – we treated Phase I patients in negative pressure rooms so that there was no danger of virus escaping into the environment, for example. But these days it’s little different to administering any other drug, other than it tends to occur in a side room away from other patients, because the general experience has been that the technology is safe and poses little if any risk to other patients, healthcare providers and other contacts, or the environment.
T-VEC Timeline
1999 – Biovex founded
June 2002 – EMA gives the go-ahead for first human trial
May 2005 – Positive Phase I/II clinical trial results announced
October 2006 – Phase II clinical trial begins
June 2008 – Positive Phase II clinical trial results reported
April 2009 – Phase III OPTiM clinical trial initiated
January 2011 – Biovex acquired by Amgen
March 2013 – Positive Phase III clinical trial results announced
October 2015 – Approved by FDA
December 2015 – Approved by EMA
Do you anticipate improvements over the clinical data reported for approval?
It’s already happening. The latest Phase III data (presented by Amgen at the joint ODAC/CTGTAC committee, which reviewed the data for the FDA in April 2015) now show that 32 percent of the treated patients achieved an objective complete or partial response, and 17 percent – not the 11 percent originally reported – had a complete response. While it is true that the survival benefit was only marginally significant (p=0.0494) for the entire study population in these updated data, the trial enrolled a very broad group of patients, from early- to late-stage, and from previously untreated patients to those who had tried many treatments. The survival data for the approximately 50 percent of patients who had earlier disease (i.e., with no disease in visceral organs) and the overlapping approximately 50 percent who were treated as first-line therapy were very compelling indeed, with a highly statistically significant effect in both cases (p<0.001) and hazard ratios favoring treatment with T-VEC of 0.57 and 0.50 respectively (1). Overall, I think the T-VEC data are highly encouraging, particularly for patients with earlier stages of disease. It was these data in particular which led to the approval of T-VEC in Europe, announced by Amgen on December 17th 2015.
How is T-VEC positioned with regard to other systemic immunotherapies?
We had long suspected that T-VEC would synergize with the checkpoint inhibitors, because T-VEC induces both an immune response to tumor antigens and inflammation in the tumor. Both of these are necessary for checkpoint inhibitors to work but they are not present in all patients. Therefore, T-VEC could be given with checkpoint blockade such that a greater proportion of patients respond and the magnitude of response is increased. In particular, complete responses following treatment with checkpoint blockade agents are relatively rare, and we expected that the rate of complete response would be increased by combination with T-VEC or other oncolytic immunotherapies. And indeed, both pre-clinical and clinical data have now shown that combinations of T-VEC and checkpoint inhibition are highly synergistic, with very high rates of response (50–60 percent) in melanoma patients when combined with either ipilimumab or pembrolizumab. Larger controlled studies, and studies of these combinations in other tumor types, are now under way. Needless to say, as the data develop I'm becoming very excited by the prospects for combined oncolytic immunotherapy and checkpoint blockade therapy, and expect these to become a cornerstone of cancer therapy.
Lessons learned?
I think we have found out that, when you are at the forefront of anything new, you have to pretty much ignore precedent.We have also found that you have to be very wary when the so-called experts tell you a path forward is impossible, with no sound rationale other than it hasn’t been done before. Instead, you have to think deeply about what your product is, what data you have and what you need to do to take it forward, and move ahead on that basis. In summary, you must forge your own path.
Robert Coffin is currently Founder, CEO and Director of Replimune Ltd, Oxford, UK.
Robert Coffin is currently Founder, CEO and Director of Replimune Ltd, Oxford, UK.















