Markers for Prognostic Progress
A combination of new biomarkers could aid decision-making in the clinical management of early-stage lung cancers
At a Glance
- Lung cancer is the most fatal cancer, causing nearly one-fifth of all cancer deaths worldwide
- To improve survival, it’s vital to diagnose and treat lung cancer early, so we need better diagnostic and prognostic biomarkers
- A combination of markers, including HOXA9 promoter methylation and blood vessel invasion, may assist with prognosis of early-stage lung cancer
- Ideally, these two biomarkers could be used in combination with a range of others to yield the most detailed picture possible of an individual’s disease
Lung cancer remains the top killer worldwide among cancers, causing nearly one-fifth of all cancer deaths worldwide (1). But not every patient with lung cancer faces the same fate; those diagnosed early have a good prognosis, with one-year net survival rates for stage I cancers at 83 percent (compared with only 17 percent for those diagnosed at stage IV). It’s clear that early diagnosis confers a strong survival benefit – and biomarker analyses that yield prognostic and treatment decision information only compounds this advantage.
Yet only about a quarter of lung cancer patients are diagnosed at an early stage, with approximately half diagnosed at stage IV. Every available piece of information counts when aiming for the best possible outcome – especially when the disease is spotted so late. The analysis and integration of different types of biomarkers is a core principle of precision oncology, as it allows us to track the potential course of a patient’s disease, and to select and individualize treatment plans as quickly and efficiently as possible. To that end, Ana Robles and her colleagues (2) have identified a combination of an epigenetic and an immunohistochemical biomarker (HOXA9 promoter methylation and blood vessel invasion, respectively) that may help inform the clinical management of patients with early-stage lung adenocarcinoma (see Figure 1).
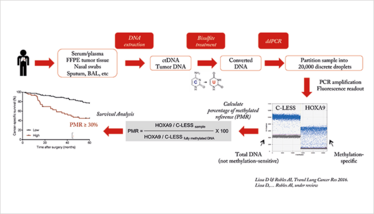
Figure 1. A schematic of the method developed by Robles and colleagues.
Prognostic promoters
Tumor tissues commonly feature alterations in DNA methylation. Moreover, specific changes in methylation are consistent across tumors, meaning that their patterns can serve to discriminate tumor tissue from its normal adjacent tissues. Concurrent methylation in the promoter regions of developmental genes (collectively known as homeobox, or HOX, genes) is one such recognized feature of lung cancer. Among these genes, HOXA9 has generally stood out for being the most differentially methylated between tumor and non-tumor tissues – and, therefore, the most potentially useful for lung cancer diagnosis and prognosis. Homeobox genes are epigenetically regulated in embryonic stem cells, which could mean that high HOXA9 methylation identifies a less differentiated chromatin state, or even subpopulations of cancer stem cells that may be responsible for recurrence and resistance to therapy. Blood vessel invasion (BVI) is a recognized prognostic factor in many cancers and identifies neovascularization of the primary tumor, which is a critical step for tumor cell dissemination and metastasis. Why measure both together? The same biospecimen can be used for both, and each is an independent biomarker, so the combination is strongly predictive of poor outcome.
Many lung cancer prognostic biomarkers have been identified through the molecular analysis of fresh-frozen resected tissues – but this type of biospecimen is rarely available in routine clinical practice. To implement a new biomarker in the clinic, you need a robust and technically simple assay that can use materials generated for routine pathology after surgical resection as an input – for example, formalin-fixed, paraffin-embedded (FFPE) blocks. FFPE tissues are generally available for the assessment of biomarkers after diagnosis and staging, and they are very useful in the discovery and clinical development of biomarkers; however, their value for molecular analysis can be limited because of low DNA quality. And so, evaluating an immunohistochemical biomarker such as BVI in parallel with an epigenetic biomarker is a valuable strategy for prognostic prediction.
Validating an assay in archival FFPE tissues facilitates its clinical translation. Droplet digital PCR (ddPCR) is especially suited to evaluate small biomarker panels because it’s fast, simple, cost-effective, and ultra-sensitive. The technique speeds up the validation of new biomarkers to move them rapidly and efficiently along the pipeline to the clinic. The process of biomarker validation requires optimization of the ddPCR reaction for sensitivity and specificity. In the case of a methylated marker, this includes reaching an acceptable lower limit of detection of a control methylated DNA fragment on the background of unmethylated DNA, to mimic the conditions likely to be found in actual samples.
Beyond the lung
Neovascularization is a common theme in many cancers, so it seems clear that BVI has applications outside of lung cancer. HOXA9 methylation has also been described in oral and esophageal cancers, so it’s possible that this epigenetic biomarker could also have further applications, but it appears to have the highest prognostic value for lung cancer, where it has wide applicability. For instance, in the minority of Stage I lung cancer patients who receive adjuvant therapy, the HOXA9 methylation assay is still prognostic. Additionally, the assay was able to provide prognostic information in lung cancer patients without a history of cigarette smoking.
At the moment, Robles is collaborating with a group interested in evaluating HOXA9 methylation by ddPCR in their cohort of lung cancer patients. And there are plans to test the performance of the assay using FFPE tissues for the prognostic classification of individuals who developed lung cancer within the National Lung Screening Trial. In the context of this large screening trial, patients were diagnosed after a positive low-dose computed tomography (LDCT) scan. This type of screening is becoming widely implemented and is helping to diagnose lung cancer at an earlier stage. Up to 60 percent of lung cancers diagnosed after a positive LDCT scan are Stage I, which is the intended target of our prognostic assay. It’s possible that, one day, the two tests could work together – LDCT scanning to detect the cancer in its earliest days, followed by methylation and BVI analysis for prognosis and to assist with treatment decisions.
Robles and her colleagues are also employing machine learning tools to integrate different omics data for individual patients with stage I lung cancer. The goal? A better understanding of the biology of the disease, so that we can identify the most informative biomarkers. In general, large prospective studies will be needed to validate the clinical utility of biomarkers. The hope is that, with novel technologies such as ddPCR and machine learning, they will be able to identify and validate biomarkers with ever-increasing speed and ease.
Ana Robles is an Associate Scientist at the National Cancer Institute, Bethesda, USA. Her research focuses on omics-based identification and functional characterization of clinically informative biomarkers of lung cancer.
- Cancer Today, “Cancer Fact Sheets: Lung Cancer” (2012). Available at: bit.ly/2rR37PJ. Accessed May 18, 2018.
- D Lissa et al., “HOXA9 methylation and blood vessel invasion in FFPE tissues for prognostic stratification of stage I lung adenocarcinoma patients”, Lung Cancer [Epub ahead of print] (2018). DOI: doi.org/10.1016/j.lungcan.2018.05.021.
Ana Robles is an Associate Scientist at the National Cancer Institute, Bethesda, USA. Her research focuses on omics-based identification and functional characterization of clinically informative biomarkers of lung cancer.















