In Good Company
What does it take to create a competitive companion diagnostic? Bharathi Vennapusa outlines her role in the development and approval of the ALK CDx – a fully-automated immunohistochemistry assay that identifies lung cancer patients who may be eligible for treatment with Pfizer’s Xalkori (crizotinib).
How did you get involved in companion diagnostics development?
After training as a pathologist and specializing in molecular pathology, I decided I wanted a career that was neither entirely basic research nor clinical practice. I didn’t know much about companion diagnostics at that time, but through a friend I learned about Ventana Medical Systems (now a member of the Roche Group), which was active in the field. Pathologists often don’t consider careers in pharma, but I became inspired by the prospect after reading a journal article by Ventana’s Chief Medical Officer, Eric Walk, which discussed the role of pathologists in the industry. I decided I wanted to get involved, so I joined Ventana as a pathologist in
companion diagnostics.
Working in companion diagnostics allows me to get involved in research that can be translated into clinical practice. Certainly, our biomarker assays can be used for research, but our main goal is to develop assays that can be used in the clinic. We all have our own reasons for joining Ventana – some may have family members afflicted with cancer, for example – but we all share a real personal interest in improving the lives of cancer patients. In reality, companion diagnostics are the cornerstone of personalized healthcare; they are critical to finding the right treatment for the right patient.
Why develop ALK CDx, given that a competing product was already available?
It’s true that Abbott was already marketing the Vysis fluorescence in situ hybridization (FISH) assay as a companion diagnostic for Xalkori. But Pfizer wanted to develop an immunohistochemistry (IHC) assay, and so they approached us. From my perspective, having worked with both FISH and IHC assays, IHC has significant advantages. With IHC, the turnaround time is faster – patients can receive results in days as compared to weeks for FISH. Part of the reason that FISH is slower is because it’s not fully automated – some manual work is required. To perform that manual work, specific training and a specialized microscope set up in a dark room are required. One can’t just read the assay from one’s own office. This contributes to FISH assays being more expensive. In contrast, the ALK CDx is more fully automated and can be validated and run in any lab that can perform IHC assays. It is less expensive, easier to interpret, and can be read by any trained pathologist with a regular microscope in a regular setting. IHC is also accessible to pathologists almost anywhere, including the EU, China, and the US. The assay was also validated by method comparison; in other words, we tested patient samples that had already been tested by FISH and we demonstrated very high concordance with the ALK CDx assay, so the quality of the data is the same as with the FISH assay. We’ve had great feedback from users; pathologists really like the assay and appreciate that they can interpret it themselves rather than via a technician.
How straightforward was the regulatory pathway?
We have found the FDA to be very helpful during product development, both with diagnostics and with drugs, and it was the same story for the ALK CDx assay. I think the encouraging data associated with new cancer immunotherapies is helping regulators rethink their strategy and guidance, which is also making them increasingly more collaborative – especially with regard to relevant companion diagnostics. Indeed, the FDA encourages diagnostic and drug companies to collaborate on strategies to exploit the many molecular markers that have been discovered. It’s a regulatory attitude that is likely related to the many unmet medical needs in oncology; at present, only a minority of cancers are treated with targeted therapies. Regulatory support for the development of companion diagnostics will help get new targeted treatments to cancer patients sooner rather than later.
All the same, when ALK CDx was approved, we all felt like a great milestone had been achieved. Everyone was excited – not just the internal team, but the entire company – because developing a good, sensitive and specific assay takes a lot of work, and submitting documentation and answering the questions posed by the regulators can be stressful. The approval was great in itself, but it also gave us confidence in our other development-stage companion diagnostics.
What challenges did you encounter?
One of the major challenges faced by companion diagnostics companies is the difficulty in procuring sufficient cancer tissue for product development. Validating the assay requires many tests and studies, which was particularly challenging because the prevalence of ALK+ lung cancer is about five percent. We had to screen thousands of patient samples to get sufficient numbers to support our ALK CDx assay development program, and it’s not always easy to get good quality samples in these quantities.
Another challenge is that, although the ALK CDx assay is very easy to interpret, pathologists still need to be trained in its use so that they can appreciate the nuances of the assay, and understand its constraints. Essentially, we need to do everything in our power to prevent the risk of a wrong diagnosis. To that end, we developed an e-learning tool for the ALK CDx assay to walk pathologists through the challenges that they might encounter when interpreting the assay in real life. Such training is an area that we intend to continue to work hard on and constantly improve.
At a Glance
Product name: Ventana ALK (D5F3) CDx Assay
Brand name: ALK CDx
Developed by: Ventana (Roche), in collaboration with Pfizer
Marketed by: Ventana (Roche)
Product Description: Laboratory immunohistochemical test that identifies whether the anaplastic lymphoma kinase (ALK) protein is present in a non-small cell lung cancer tissue sample. A positive result indicates that the patient may be eligible for treatment with the Pfizer drug crizotinib (Xalkori).
Approval status: Approved in the USA (June 2015), Europe (October 2012), and China (Sep 2013).
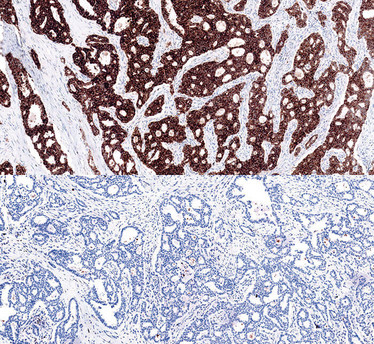
Positive (top) and negative (bottom) case of lung tissue stained for ALK with Ventana ALK (D5F3) CDx Assay.
What changes would you like to see in the companion diagnostics industry?
One of the greatest opportunities for change lies in the economics of companion diagnostics. At present, diagnostics are not always reimbursed – and when they are it is at a much lower rate than the related therapeutic. This holds back funding for diagnostics development. I’d really like payers to develop a better understanding of what we are doing. I’d also like the medical community to better appreciate what pathologists do. Pathologists are the ones enabling the diagnosis, and the tests pathologists do determine what treatment the patient will receive. Companion diagnostics essentially help the patient find the right treatment, which also means reducing the risk of exposing the patient to unnecessary treatment. Yet the funding for companion diagnostics development, and the incentives for commercialization, are relatively low. We need to educate key stakeholders about the value of these products – not just pathologists, but also payers, government bodies and private insurance companies.
I’d also like to see an honest dialogue between stakeholders, including the regulators, around the issue of obtaining sufficient cancer tissue to validate companion diagnostics. I feel that there is room for improvement in that area. In fact, communication in general is an area for constant improvement. We certainly have a close relationship with the regulatory bodies in the US, China, and Europe, but we want to improve and extend that further. Likewise, I also think we need to continue to grow our relationships with pharma companies and with independent pathologists. Getting feedback from experts outside the company – for example, on how we can improve training in assay interpretation – is critical. We’ve learned a lot of lessons from the ALK CDx assay, which we’ve already started implementing in the development of newer companion diagnostics.
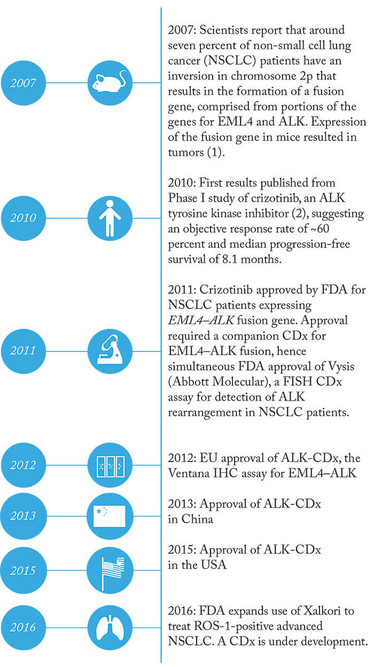
Timeline: Crizotinib and Companions
Any thoughts on the future of companion diagnostics?
Over the last four years I’ve seen explosive growth in companion diagnostics development. There were only one or two projects when I started, but now we are working on more than 10 such projects at any one time.
In all our companion diagnostics projects, especially the IHC-based assays, we expect to see more success, but also more assay complexity. For example, while some drugs may be safe and effective when prescribed on the basis of assaying a single biomarker, in the future we may need to base a prescription on two or more biomarkers, which implies presentation in a multiplex format. Accordingly, we are developing a multiplexing capability that can test for multiple markers on a single slide. This resource may also help address the difficulty in procuring sufficient tissue specimens from cancer patients, which is being exacerbated by the trend to use less invasive procedures. So if, as seems likely, diagnostics developers have much less tissue to work with in the future, next-generation technologies like multiplexing may be essential to be able to fully exploit what is available. In addition, we may need to develop digital pathology techniques, PCR, next generation sequencing, and bioinformatics tools to help decipher the data output.
By expanding the use of new, relevant technologies in companion diagnostics, by incorporating additional guidance from regulatory agencies, and by closely collaborating with regulators, drug developers and diagnostic companies, I believe society will quickly start to see the benefits of next generation companion diagnostics. And I am very excited to be part of this evolving story.
- M Soda et al., "Identification of the transforming EML4-ALK fusion gene in non-small cell lung cancer", Nature, 448, 561-566 (2007).
- DW Kim et al., "Results of a global phase II study with crizotinib in advanced ALK-positive non-small cell lung cancer (NSCLC)", J Clin Oncol, 30, 7533 (Suppl) (2012).
Bharathi Vennapusa is Principal Pathologist I and Manager, CDx projects at Ventana Medical Systems, Inc., Tucson, AZ, USA.















