
Lost in Translation?
Scientists from opposite ends of the translational spectrum have teamed up to help solve a pressing problem in Alzheimer’s research. By creating a human equivalent to the water mazes used in rodent studies, they hope to allow easier comparison of data from animal and human studies.
The importance of collaboration when it comes to successful translation is undeniable, and yet partnerships between basic and clinical researchers remain relatively uncommon. There is often little dialogue between the scientists working with mouse models of a disease and the clinicians who attempt to apply the findings to human patients. Without clear lines of communication between these two groups, how do we know if their methods – and conclusions – are comparable?
A recent project brought together scientists from across the divide in a collaborative effort to create a (virtual) human version of the classic rodent test of navigational learning – the Morris water maze (1). Here, we speak with study collaborators Kate Possin, Steve Finkbeiner, and Pascal Sanchez (see profiles on page 21) to find out how harmonizing cognitive tests could speed up clinical translation in Alzheimer’s.
Why does translation from animal models to humans pose so many problems?
Steve Finkbeiner: There are a lot of different ways to answer this question. One is that there is a limit to the extent that you can model a human disease in mice; there are many differences in the way mice and humans metabolize drugs, and translating between two different species is never going to be easy. But another factor could be the way that tests are carried out or how data are analyzed. With this project, we wanted to identify and try to minimize those differences.
Pascal Sanchez: We could spend hours talking about the translational challenge in moving from mouse models to humans. In the past, maybe people have put too much faith in animal models. I think we need to take a more nuanced approach. Animal models are useful, but I believe we need to start thinking about them in a different way, rather than trying to translate the discovery of a given drug in a mouse directly to humans.
I think another problem is that the media likes papers that make a big splash. They want a huge discovery in mice to immediately translate to humans. In reality, we all know that animal models only recapitulate some aspects of the disease. It’s a much more complex picture in humans.
How did the collaboration begin?
SF: The genesis of our journey was a large philanthropic gift in 2009. We could have spent the money doing more basic research, but this particular donor had two sons at risk of neurodegenerative disease, and it seemed like a good opportunity to focus on moving our discoveries closer to the clinic. Part of that strategy involved partnering with pharma, which took me on a very steep learning curve over the obstacles for clinical translation. There is a perception amongst companies that doing drug development in neurologic diseases is incredibly risky, partly because animal models often fail to predict outcomes in clinical trials.
Then we got a gift from another philanthropist, who had already been supporting the Memory and Aging Center (where Kate works), and was keen to foster collaboration. We were looking for important aspects that would bring our two groups together – and help make the translational pipeline more predictable – which led us to Kate’s work.
Kate Possin: My group was looking into testing navigational impairment in patients with Alzheimer’s disease. We wanted to find a way to measure navigational learning, which you obviously can’t do by using pencil and paper tests. You need real-world paradigms. You could do something like lead patients around the hallways of a hospital to see if they find their way, but that is not a very controlled environment. A standard test for cognitive impairment studies in rodents – and therefore studying mouse models of neurological diseases – is the Morris water maze. To that end, we had been working with computer programmers at Microsoft Research to develop a virtual water maze that could be applied to human patients.
SF: We were really lucky to find that Kate was already thinking about these questions, and looking into innovative work on the water maze. That led the way for Pascal and others – who had done some beautiful work in mouse models – to generate comparable data sets.
PS: The goal was relatively simple: to create a test in which we could really engage the hippocampus in both humans and mice. Ultimately, that would enable us to better predict efficacy across species – and therefore improve the chances that a treatment that works in mice will also work in humans.

Why is navigational impairment important in Alzheimer’s disease?
KP: In humans with Alzheimer’s disease, getting lost is one of the early symptoms, because remembering how to get somewhere relies on the hippocampus – the first area of the brain to show damage from Alzheimer’s pathology. To test a treatment for Alzheimer’s disease you ideally want to target patients in the earliest stages, so you need a cognitive measure that is sensitive to early cognitive changes. Another reason navigational impairment is so compelling is that there is a huge body of research on the anatomy of navigation learning from years of rodent studies. Being able to measure the navigational impairment caused by Alzheimer’s disease in both species gives us the potential to directly compare results across species, including studies of drug efficacy.
Human analogs of the Morris maze have been developed in the past, but there are a number of problems. Protocols vary and don’t always match well with the mouse version; it was unclear which performance measures were most sensitive; and the statistical analyses used often fell short. In this study, we wanted to address those limitations.
What were the challenges in working across disciplines?
PS: As with the start of any new collaboration, it took a little time just to understand each other. We’re all in the fields of neurology and neuroscience, but making analogies between the mouse and human tests wasn’t easy. The way we measured different aspects of performance was quite different, so it took time to harmonize. Now, if we started on a different project together, it would be much faster because we understand each other better.
SF: At Gladstone, we actually tried many years ago to meet regularly with the Memory and Aging Centre to come up with a common language, but it failed. We had several meetings to reach that goal, but it was like our groups were from Mars and Venus!
I think one of the key reasons why our collaboration worked where others had failed was down to the people involved – Kate and Pascal are highly motivated. And the philanthropists’ support was also crucial, because it enabled us to work at an interface where grant funding is very scarce; agencies are generally devoted to either pre-clinical or clinical work, but not the translational link between the two. In essence, the philanthropic funding helped form the glue to stick us together.

The Collaborators
Kate Possin is an assistant professor of neuropsychology at the Memory and Aging Center within the University of California, San Francisco (UCSF) Department of Neurology. Her work focuses on understanding the neurological basis of cognitive deficit, including developing new tools to measure cognition in patients with neurodegenerative diseases such as Alzheimer’s.

Steve Finkbeiner is a professor at UCSF and Director of the Taube/Koret Center for Neurodegenerative Disease Research at the Gladstone Institutes. His lab strives to understand the molecular mechanisms involved in learning, memory and neurodegeneration, while the Taube/Koret Center aims to take discoveries from the lab and develop them into viable drug candidates that can be taken forward by industry partners.

Pascal Sanchez is a neuroscientist at the Gladstone Institutes. His aims are to discover and develop new drugs for neurodegenerative diseases in collaboration with industry partners, validate animal models of disease, and develop translatable cognitive tests.
What were the first steps?
KP: After meeting Steve, I got a tour of the lab where they evaluate navigation learning in rodents. I talked with the staff who carried out the tests to make sure I really understood how it was administered in mice.
The other important part of the early collaboration was regularly meeting with people from Steve’s group – which Pascal joined soon after – to talk informally about how we measure cognition across species and where there were links. As Pascal and Steve mentioned, we had a lot of really interesting conversations and spent time learning each other’s languages, before we honed in on this particular project.
SF: I remember Kate’s first visit. She was surprised at how often we use negative reinforcement for the mice in the tests, because of course that isn’t something we do in humans. You can’t put people into a swimming pool and make them swim around until they find a platform.
The Morris Maze: Mouse vs Human
Mouse
Devised 30 years ago by Richard Morris, the Morris water navigation task (Morris maze) measures spatial learning and memory in rodents.
- The mouse is placed in a circular pool, filled with water made opaque by powdered milk or nontoxic paint.
- To escape the water, the mouse must locate a platform.
- At first, the platform is visible above the water -the animal learns that swimming to the platform means escape.
- Now the water level is raised so the platform is hidden – the animal must navigate to it from memory.
- Visual cues (e.g. posters) are arranged around the room outside the pool, so the mouse can ‘triangulate’ its position.
Human
Previous Morris maze analogs in humans have included real-space, 2D and virtual environments, but protocols vary and do not always correlate between species.
- The authors set out to discover how well the test translates between animals and humans.
- They developed a simple driving simulation, displayed on a computer monitor.
- The goal for participants is to find “buried treasure” in a circular area.
- The subject is first asked to drive to a visible target, with no other cues present.
- Then, the target is hidden and trees, houses and other structures are added in the distance, to provide visual cues.
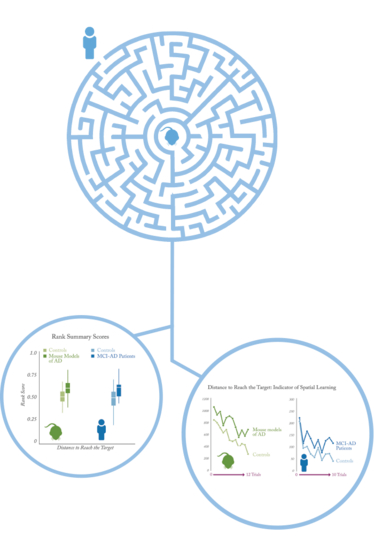
How comparable were the tests?
KP: The human and rodent maze tests do seem to be comparable. Early-stage Alzheimer’s patients and transgenic mice expressing amyloid precursor protein showed a similar level of impairment on the test, compared with healthy controls.
The biggest difference is that in the mouse model, there’s a set of trials where the mouse has to learn to swim over to a visible platform. In human tests, we can simply tell the patient to drive to the target, so even patients with a high degree of impairment can do it, while the mice suffering neurodegeneration struggled with this task.
PS: The task is more complicated for mice. We cannot tell them what they have to do, so they have to discover it by themselves. The incentive to perform is also different. For mice, it is a stressful situation. You put them in water and they have to escape. Human subjects are in comfortable chairs, at room temperature. They want to perform well, but it’s not the same as the stress in the mouse model, so the incentive to perform is different.
One thing that I learnt while working with clinicians is that it’s not as easy to tweak the experiments in humans as it is with mice models. But we have been thinking about ideas to increase motivation in human subjects. For example, they could be given bitcoins, which they will lose if they fail to perform to a certain level.
That is a huge advantage of clinicians and basic researchers working with each other: coming together to identify a disparity and figuring out the best way to adjust for it. Something that could have easily been adjusted in the animal model might require a complicated work-around in human tests or be missed completely – and that’s why collaboration is so important.
What can others learn from your work?
PS: Our goal was to provide not just a description of our results, but real-world recommendations for other researchers in the field. The Morris water maze has been extensively used in mice and rats, but unfortunately people are not necessarily using it correctly or analyzing the data in the right way. So in our recent paper, we provide a number of recommendations on the best way to use the test in humans and animals, including appropriate sample sizes to be able to detect disease-related differences.
KP: When I was showing the data in human patients to Pascal and the others, I was very concerned because it looked so messy. There was a lot of trial-by-trial variability, which led to concerns over the way these types of data are typically analyzed. Then Pascal showed me the mouse data, and it turned out to be just as messy. I thought I was doing something wrong with the humans, but actually we were getting very similar data.
We then wanted to find the cleanest possible way to analyze the data, so we worked with a couple of statisticians to explore different analyses that might be appropriate and powerful enough to detect group differences and, in future, maybe even drug effects. Typically, Morris water maze analyses use repeated-measures ANOVA, but as we – and others – have pointed out, this method violates some key statistical assumptions. We present a rank summary score method that avoids those problems but is still powerful. It also happens to be the easiest one to apply, so it doesn’t require a strong statistical background.
What have you learnt from cross-disciplinary collaboration?
KP: We achieved results and solved problems that we might not have done if we worked separately. It was a great experience that not only benefitted the project, but also helped us all personally.
SF: I’d add that it is tough! It really did feel like we were speaking separate languages, and made me realize that our respective worlds are pretty different. It takes real commitment from the groups involved to work together on an important problem.
PS: It’s hard, but it’s also extremely rewarding. We would all like our discoveries to be translated and collaborations like this help us to be more involved in that process. These types of projects shouldn’t be outliers and, given that the UCSF Memory and Aging Centre is right next door to the Gladstone Institutes, we have no excuse! We have several collaborative projects ongoing, and hope to set up more in the future.
KP: To continue the Morris maze work, we hope to explore whether the test can be used to measure drug efficacy. Another important piece of research would be to compare the test to other cognitive measures that are typically used in Alzheimer’s drug trials, such as the Alzheimer’s Disease Assessment Scale for Cognition (ADAS-Cog).
- KL Possin et al., “Cross-species translation of the Morris maze for Alzheimer’s disease”, J Clin Invest, 126, 779-78 (2016). PMID: 26784542.
Pascal Sanchez is a neuroscientist at the Gladstone Institutes. His aims are to discover and develop new drugs for neurodegenerative diseases in collaboration with industry partners, validate animal models of disease, and develop translatable cognitive tests.
Steve Finkbeiner is a professor at UCSF and Director of the Taube/Koret Center for Neurodegenerative Disease Research at the Gladstone Institutes. His lab strives to understand the molecular mechanisms involved in learning, memory and neurodegeneration, while the Taube/Koret Center aims to take discoveries from the lab and develop them into viable drug candidates that can be taken forward by industry partners.
Kate Possin is an assistant professor of neuropsychology at the Memory and Aging Center within the University of California, San Francisco (UCSF) Department of Neurology. Her work focuses on understanding the neurological basis of cognitive deficit, including developing new tools to measure cognition in patients with neurodegenerative diseases such as Alzheimer’s.















