A Rare Delight
Anthony Quinn shares the story behind the development and approval of sebelipase alfa (Kanuma) – the first and only enzyme replacement therapy for lysosomal acid lipase deficiency (LAL-D).
What led you into drug development?
I trained in internal medicine and dermatology in the UK, and followed that with a PhD in molecular oncology and a post-doc in genetics at the University of California, San Francisco, before becoming Professor of Dermatology at Bart’s in London. Translational medicine wasn’t particularly fashionable in those days, but drug development fascinated me, so it wasn’t long before I moved to pharma – first AstraZeneca, then Roche in California. After Roche closed their Palo Alto site following the acquisition of Genentech, I decided that I wanted to apply my drug development know-how in a small company setting. I was intrigued by a biotech called Synageva which had technology from the University of Georgia for producing recombinant proteins in egg white (EW) using transgenic hens. To me, this seemed a very promising approach that might facilitate the manufacture of more stably glycosylated proteins. I became Head of R&D and Chief Medical Officer at Synageva, in 2009 and oversaw the program to develop recombinant sebelipase alfa for LAL-D.
LAL-D is a chronic, progressive, inherited disease caused by the deficiency of an enzyme that breaks down lysosomal lipids. Lipids build up in the tissues over time; the liver can be especially badly affected. In infants with the most rapidly progressive form of the disease, death typically occurs by six months of age. In older patients, progression of the disease (historically known as cholesteryl ester storage disease) leads to cirrhosis and other complications in childhood or later in life, with a shortened lifespan.
Why did Synageva decide to focus on LAL-D?
Originally, the EW technology was being applied by a company concentrating on biosimilars and biobetters. This company was refinanced and renamed Synageva following the appointment of a new CEO, who envisioned that the technology platform had potential for developing new enzyme replacement therapies and other innovator drugs, rather than just making copies of existing products. We decided that there was a significant opportunity to develop a first-mover product in LAL-D for several reasons. Firstly, it was one of few remaining lysosomal storage disorders that did not appear to have brain manifestations. That was important because lysosomal enzyme disorders with brain involvement are challenging to treat using intravenously injected enzymes that can’t cross the blood–brain barrier. Secondly, there were no effective therapeutic options. Although there had been some earlier preclinical studies looking at enzyme replacement, the results were not encouraging enough to progress into the clinic. Thirdly, in 2007, there was a genetic epidemiology study by Muntoni that examined the frequency of disease carriers for LAL-D in the German population. Muntoni’s results suggested (based on the Hardy–Weinberg equation) that LAL-D should be much more common than the literature suggested. This finding and our own analysis of the level of disease awareness and the clinical manifestations led us to conclude that many patients with LAL-D were undiagnosed.
One of LAL-D’s complications is cirrhosis, but when hepatologists saw cirrhosis in children or in adults, they were not typically thinking of this disease. They would think of more common causes of cirrhosis like viral hepatitis (B/C), nonalcoholic fatty liver disease, and – in adults – alcohol-related liver disease. If they drew a blank with those, they would consider less common causes such as autoimmune hepatitis, Wilson’s disease, and alpha-1 antitrypsin deficiency. The possibility of LAL-D is often not considered. Another manifestation of the disease is very high LDL levels, but specialists were often not aware that LAL-D could cause elevation of cholesterol to levels that resembled familial hypercholesterolemia, so they didn’t test for LAL-D as part of the differential diagnosis. It’s a real gap in the textbooks. We found instances of children with LAL-D whose disease had progressed to the point of liver transplantation, and yet they remained undiagnosed.
The issue of low disease awareness and under-diagnosis of rare diseases continues to be problematic and often only becomes apparent when new therapeutic approaches start to emerge. Communicating to physicians who diagnose and care for patients with rare diseases takes a long time, and then it takes a while for physicians to incorporate new learnings into their practice. I don’t know what the current situation is (I’m no longer directly involved in activities supporting improved LAL-D awareness), but when I was at Synageva, we found that the more physicians we spoke to, the more cases of undiagnosed or misdiagnosed patients we uncovered. We also found that many physicians underestimated the impact of the disease; for example, we spoke to a pediatric hepatologist who remembered an LAL-D child being fine, but subsequent follow-up revealed that a liver transplant was needed shortly after the patient was transferred to the care of an adult hepatologist. Sadly, such stories are not isolated.
Tell us about the technology behind sebelipase alfa
In order to make a transgenic hen that can produce a therapeutic protein you make a small hole in the top of a fertilized hen egg and inject a retroviral vector encoding the therapeutic protein. The vector inserts itself into the hen DNA, resulting in germline transmission of the transgene over many generations, and stable expression of the therapeutic protein in EW at commercially viable levels. There are a number of potential advantages of producing therapeutic proteins in EW. There is almost no contamination with DNA, in contrast to cell culture approaches where media contain dead cells and DNA that can complicate the purification steps. Moreover, there are very few proteases in EW – in fact, there are very high levels of anti-proteases – so it’s a friendly milieu for a number of proteins.
The transgenic hen system offers one other potential advantage – stability. One of the challenges of biologics, particularly glycosylated proteins, is that glycan structures can change during manufacture. Why? Because you are using cell lines based on transformed cells, which are genetically and epigenetically unstable. The transgenic hen system, however, allows production of recombinant glycosylated proteins in normal cells that are less likely to show this type of instability. And with cell culture, although you can make protein quickly at small scale (for example, for lab experiments), scale-up can cause changes in glycan structure. With the EW production system, it may take a little longer to set up a production line, but once it’s in place the scale-up process is more straightforward, with less risk of unexpected changes in product glycosylation. One other benefit of the EW platform is that the infrastructure costs of having an extra upstream manufacturing facility are relatively small compared to cell culture-based manufacturing. We were able to have redundant facilities in different parts of the country, which helps mitigate any supply chain risk and gives you instant extra capacity, if required. Companies that use cell culture-based manufacturing may not be able to provide this redundancy as early because of the costs of cell culture facilities.
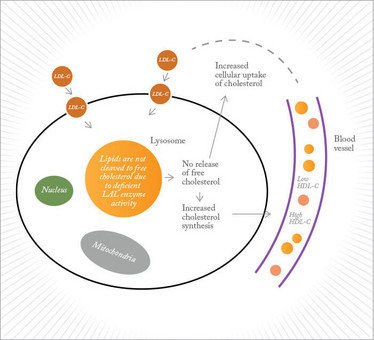
Figure 1. Effect of lysosomal acid lipase deficiency on cholesterol metabolism.
It’s an unconventional system – were there any regulatory hiccups?
There was one established precedent for production of recombinant proteins in transgenic animals: an antithrombin product, called ATryn, which is harvested from the milk of genetically modified goats. But our system was quite different, and we had to do a lot of trail-blazing to move sebelipase alfa forward. It was helpful that we had an innovator product that addressed a disease of very high unmet medical need – the regulatory focus was more on the product in the context of the disease, and less on the differences between EW manufacturing and more conventional manufacturing platforms. We were judged on the therapeutic protein, the toxicology data, and the clinical data, rather than being measured against a pre-existing product.
On the whole, we found that both the European and the US regulators were supportive. Of course, regulatory authorities have a big responsibility; if something goes wrong, people will ask questions – and in the US, they could end up in front of a government committee. Regulators are very data driven, and that means jumping cleanly through all the hoops and ticking all the boxes to get a product approved. We went through reviews of clinical trial design and data and reviews of the manufacturing process; also, because this was for a transgenic system, an additional review body had to be involved – the Center for Veterinary Medicine (CVM).
With hindsight, an important difficulty with the regulatory authorities may have been related to the paucity of information and the accuracy of descriptions of LAL-D in medical textbooks. Regulatory perceptions of a disease are usually based on the literature; if the literature is wrong (or lacking), the perceptions are inaccurate or underdeveloped. In particular, we found that the perception of the disease in affected children, young adults and older adults in the textbooks was not consistent with emergent insights from our clinical program, which made the process difficult at first. Indeed, it took quite some time to generate new information and to change perceptions.
What did sebelipase alfa approval mean for patients?
Before sebelipase alfa, there wasn’t really a standard of care; few clinicians were diagnosing cases or thinking about the disease. Affected children and adults who were diagnosed would get put on statins – and have a liver transplant if the disease progressed. For affected infants there was little that could be done except tell parents that their baby had a rapidly progressing disease and would die. In some centers, there were attempts at bone marrow transplantation, but they usually failed.
In early 2011, I witnessed the huge impact that sebelipase alfa could have first-hand. We had received permission to commence the first studies in patients; in Europe, that included infants with the most rapidly progressive presentation of the disease. But before the trial site was active, one of our investigators diagnosed a baby boy with rapidly progressing LAL-D. This physician knew we had very compelling preclinical data and that we were about to start the clinical trial – but also realized that, if we went through the required administrative process, it would be too late. He filed a request under the French compassionate use program that was supported by the hospital ethics committee and the French regulators.
This turn of events turned out to be a seminal moment for LAL-D patients – the drug worked! Before treatment, the baby had stopped growing and was expected to die from liver failure and other complications. With sebelipase alfa, the baby started growing again – exactly as we’d expect from the preclinical data. Now five years old, the boy goes to school and is doing well. But if we had been two months later or we hadn’t filed our regulatory documents in Europe, he would have died before he reached six months. The outcome was incredibly rewarding and motivating for the whole team. It also underlined to all of us why it was critical to move quickly.
And the Synageva program really did move quickly. The first preclinical data were generated in the second quarter of 2010 and we had Phase III data four years later in July 2014; we submitted the BLA on October 23, and received approval in 2015. Such a compressed schedule was possible firstly because we had a talented, focused team, and secondly because people worked incredibly hard. I’ve never worked with such a dedicated team. During the BLA submission, we were working days, nights and most weekends. A lot of that motivation came from awareness of cases like the one I just described, and from a common understanding that by doing things efficiently and effectively, we could really make a difference. It was exhausting and draining, but it was also energizing, because we could see that our efforts were translating into meaningful perceptible progress.
Did you ever think that you might not succeed?
I’ve learned that all drugs face some challenges during development. The key to success is to identify challenges and hurdles in advance, so that when they arise you’ve already got some potential solutions available. In my experience I have seen a number of promising drugs fail in development because of a failure to proactively manage emergent challenges during the drug development process. For example, if we hadn’t recognized that LAL-D was under-diagnosed and if we hadn’t put huge effort into physician outreach and disease awareness very early on in the program (including improvement of the diagnostic testing landscape) then we wouldn’t have identified patients for our Phase III trial. Fortunately, our CEO understood the rare disease environment and realized that we had to spend money raising awareness because that would be an important factor in our success. It could so easily have been very different.
Conventional market research for rare diseases can be very misleading and can stymie innovation. For LAL-D we know that other companies assessed the opportunity differently. For a low-awareness disease that is likely to be substantially under-diagnosed, market research based on historical and/or current paradigms will underestimate patient numbers for LAL-D. Sadly, for some diseases, that situation can really stop a program in its tracks. I remember giving a lecture at one of the big children’s hospitals in the US. The head of pediatric hepatology told me that LAL-D was so rare that they very rarely made the diagnosis. Two months later, she called to say they had found three cases...
At a Glance
International non-proprietary name: Sebelipase alfa
Brand name: Kanuma
Developed by: Synageva (until 2015)
Marketed by: Alexion (acquired Synageva in June 2015)
Drug class: Lysosomal enzyme
Approval status: Approved by European Commission (September 2015) and FDA (December 2015) for all patients diagnosed with LAL-D.
What’s next for you?
I’d like to leverage the skills and knowledge I have acquired over my career, including my tenure at Synageva, particularly with respect to supporting early-stage concepts emerging from academia. It was a remarkable journey and learning experience.
Changing the perception and treatment of a disease – and transforming the outlook for LAL-D patients – was incredibly satisfying. And that’s the attraction of translational medicine, of course. When I moved from academia into industry, that kind of step wasn’t as fashionable as it is now, but I really loved being in a position to take a new molecule and move it forward in a safe and thoughtful way. It’s a really exciting area of science and extraordinarily rewarding.
Anthony Quinn is Founder at IDBioPharm Consulting LLC.















