
The Second Coming of Daclizumab
Daclizumab (Zinbryta) has recently been approved by US and European regulators for the treatment of relapsing multiple sclerosis. In fact, it’s not the first foray onto the drug market for the CD25 antibody – in 1997, the drug was approved to treat transplant rejection, only to be withdrawn 12 years later for commercial reasons.
Here, Thomas Waldmann, Co-Chief of the Lymphoid Malignancies Branch at the National Cancer Institute, shares a translational odyssey – from chance discovery to welcome return.
How did you come to discover anti-Tac, the precursor of daclizumab?
The story starts over 35 years ago, not long after Georges Köhler and César Milstein published their hybridoma technique for the production of antibodies. We wished to use a monoclonal antibody to CD4 for our research into T-cell activation. But the existing antibody was embargoed, so we decided to make our own. In 1979 Takashi Uchiyama and I used a cell line isolated from a patient who was diagnosed with Sezary T-cell leukemia but who in retrospect had human T-lymphotropic virus 1 (HTLV-1)-associated adult T-cell leukemia. That misdiagnosis turned out to be very relevant, because the virus forces T cells to overexpress IL-2 and IL-2 receptor. So the cell line was not only CD4-positive, but also overexpressed IL-2, although none of this was known at the time.
Initially, we were disappointed to find that the antibody we produced did not react with CD4, or indeed with the majority of normal cells. It was rejected as a universal negative at the First International Workshop on Human Leucocyte Differentiation Antigens. However, by the second workshop, activated as well as resting lymphocytes had been added to the panel, and the antibody was shown to react with activated T cells, but not resting B cells and monocytes. We realized that the pattern of reactivity was similar to that being described for the IL-2 receptor. The link was confirmed when we found that high doses of the antibody, named anti-Tac, blocked IL-2 binding by targeting the IL-2 receptor alpha chain, now known as CD25.
When did you realize it might be clinically relevant?
Looking closely at the expression of IL-2 receptor in the body, we began to realize that it was expressed by a number of cells that one might wish to get rid of. It was highly expressed during allograft rejection, autoimmune diseases like multiple sclerosis, leukemia and Hodgkin’s disease. We began to experiment with treating animal models of these diseases with our antibodies. Our initial focus was allograft rejection and we found that anti-Tac prolonged the survival of transplanted organs in non-human primates. With the help of the NIH biopharmaceutical development program, we made mouse anti-Tac under GMP and trialed it in patients, but we soon realized this was far from ideal. The problem was, it was a mouse protein – it had a half-time of 40 hours and was immunogenic. We needed a human protein so we teamed up with Cary Queen and his newly established company Protein Design Labs (PDL), to humanize the antibody.
How straightforward was translation from there?
The science had a relatively smooth, continuous development, but business issues often caused delays. We administered the drug first in patients with adult T-cell leukemia and those receiving kidney transplants, and the results were encouraging enough to capture the interest of Hoffmann-La Roche, who licensed the antibody from PDL. They made the humanized version under GMP and called it daclizumab (trade name Zenapax). First in small preliminary trials and then in ever-larger blinded trials, it was successful in reducing rejection episodes. I could not convince the company to treat patients more than once a month for five months. The antibody works in multiple ways, but one of the big ones is to prevent IL-2 (a growth factor) from “seeing” its receptor and promoting T-cell development. If the effect was to be maintained, I felt the patient needed to continue the drug indefinitely. But antibodies were expensive to make, and the company felt that payers would not cover more doses. At one point, Hoffmann-La Roche acquired a company making another anti-rejection drug and considered terminating the daclizumab program. It can cost a billion dollars to bring a drug to market, so these business decisions are not simple. You don’t see these types of events in the literature, but they can have a huge impact on whether a new drug is released.
How did you feel when the drug was finally approved to treat transplant patients in 1997?
The approval was the first in the world for a humanized antibody. There was a huge party to celebrate the approval and that was fun, but the real reward was to see the benefit in people. I’m an MD, so I look to the clinic for my motivation. Research is a bit like planting an orange tree – you wait and wait, and hope that someday you’ll be able to harvest the fruits of your labor.
What other indications for daclizumab did you investigate?
There were several other areas where we thought the antibody might be useful. We joined Bob Nussenblatt at the National Eye Institute to evaluate the drug in patients with uveitis, and found that daclizumab could replace the currently used chemotherapeutic drugs. Unfortunately, there was no interest from industry in this indication. We also studied a disease with similarities to multiple sclerosis – tropical spastic paraparesis. It is caused by the retrovirus HTLV-1, which also causes adult T-cell leukemia. Again, daclizumab was associated with benefit, but the disease is exceedingly rare in the US, occurring mainly in the Caribbean basins, sub-Saharan Africa and Japan, and sadly it didn’t fit into any business plan.
At a Glance
International non-proprietary name: Daclizumab
Brand name: Zinbryta
Previous name: Zenapax
Developed by: Thomas Waldmann (NCI) discovered mouse antibody. Humanized by PDL BioPharma.
Marketed by: Roche for transplant rejection and respiratory indications, and later by Biogen and Abbvie for multiple sclerosis.
Drug class: Monoclonal antibody
Approval status: Approved by FDA for acute transplant rejection in 1997. Approved for adult patients with relapsing multiple sclerosis by EMA in April 2016 and FDA in May 2016.
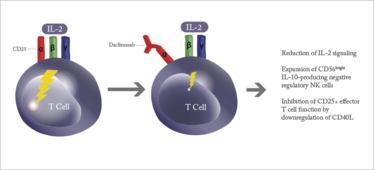
Mechanism of action.
What about multiple sclerosis?
We did some work in the early 2000s testing daclizumab in multiple sclerosis patients, with NIH colleague Bibiana Bielekova. We started with nine patients and assessed them for new MRI lesions. For the first two or three weeks, nothing happened. But then we saw a major reduction in new lesions. More studies were carried out in this and other groups, and the findings were confirmed. Biogen partnered with PDL to develop the drug for multiple sclerosis and together they sponsored a larger trial of daclizumab alongside beta interferon, again with encouraging results.
By then, six different NIH institutes and others around the world were using the antibody for a range of indications, from cancer to islet cell transplantation. And then, all of a sudden in 2009, Hoffmann-La Roche withdrew the drug from the market due to falling profits. Neither doctors nor researchers could get the drug for a time, even we who had discovered it. There was a feeling amongst some that monoclonal antibodies were passé.
Luckily, since PDL held the patent, the work in multiple sclerosis was able to continue and it was gradually evaluated in larger groups, eventually enrolling 1800 patients for two years. By now in a subcutaneous formulation, the drug was administered every 3–4 weeks, again with a significant reduction in new lesions. The results looked good, but the field of multiple sclerosis has many candidate drugs and there was always an element of suspense: would they take it to completion?
The drug was approved for the treatment of relapsing multiple sclerosis in Europe in April 2016, and in the US a month later.
It’s been a long journey...
There were 16 years between our production of the antibody, and the first FDA approval. And 35 years between the discovery of the drug and the second approval. It felt like there was a long period of eclipse, followed by a renaissance. It’s certainly been an odyssey – and something of a detour from my primary focus on cancer research.
Will we see daclizumab approved for other indications?
I think there is potential to expand the indication. We had some good results in a non-human primate model of rheumatoid arthritis, for example. But it’s a crowded market and the development of a drug is a multi-million dollar operation – you need an advocate who is willing to push development forward. But now that the drug is again available for purchase, I think we will see off-label use for conditions such as uveitis.
When you started, did you realize how big immunotherapy would be?
There was a long period where immunotherapy was ridiculed – it was considered a sort of witchcraft. Now, whether you read the New England Journal of Medicine or the Wall Street Journal, everyone is talking about immunotherapy. Monoclonal antibodies were the first areas of success, as compliments to chemotherapy or other agents. Now, checkpoint inhibitors are also having a major impact on cancer outcomes, and I think that there will almost certainly be a checkpoint inhibitor included in the panel of drugs for every cancer in the future.
If checkpoint inhibitors are targeting the brakes of the immune system, I am currently studying the gas pedal –IL-15 – which dramatically upregulates NK cells and activates CD8 cells to fight cancer. But administering IL-15 alone would be like driving a car without a steering wheel – it doesn’t have specificity for the tumor. We are now working with a combination of agents to increase antibody-dependent cellular cytotoxicity. I see the next era of cancer research as being a great expansion of immunotherapy. Continuing with the car analogy, we will be able to control the gas pedal, the brake release, and the steering.
Thomas Waldmann is Co-chief of the Lymphoid Malignancies Branch at the National Cancer Institute.















