The Best Treatment
Is gene therapy the answer to an untreatable form of macular degeneration?
Most of the time, when you diagnose a patient with neovascular AMD, there are several management strategies that should help slow progression and retain vision – and there are many more in the research pipeline. But when a patient with Best disease (vitelliform macular degeneration) presents, it’s a very different story; currently, no medical or surgical management can help.
Now, a group from the University of Pennsylvania, Philadelphia, have unraveled some of the mysteries surrounding the pathology of Best disease – and developed a gene therapy that successfully treats the canine form (Figure 1) (1). Karina Guziewicz and Artur Cideciyan, lead authors of the study, tell us more about their research, and how they’re hoping to translate their work into the clinic...
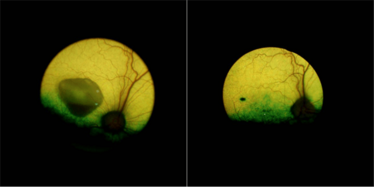
Figure 1. An eye from a dog with the canine equivalent of Best disease before (left) and five years after (right) receiving subretinal AAV2-mediated BEST1 gene therapy. Before treatment, a sizeable lesion was present (the black mark visible on the treated eye is the injection site). The group demonstrated that their gene therapy approach corrected advanced vitelliform lesions as well as light-modulated micro detachments. Credit: University of Pennsylvania.
Why tackle Best disease?
Although monogenic causes of vision loss are relatively rare – estimated to be about 1 in 1,500 to 4,000 – they allow deep understanding of pathophysiology and evaluation of gene-specific treatment strategies. Spontaneous mutations in the canine BEST1 gene have been identified in multiple dog breeds. We have found that the phenotype of canine BEST1-associated retinopathy recapitulates the full spectrum of clinical, molecular and histological features observed in patients with BEST1 mutations. These striking phenotypic similarities allowed us to gain valuable insight into the natural history of the disease and to pinpoint molecular parallels, which laid the groundwork for the development of lasting gene therapy.
Any surprising findings?
Amazing similarity of the retinal phenotype between the human and canine macular degenerations was a nice surprise. We found that the canine clinical picture evolves over many years and progresses through various well-defined stages, accurately recapitulating the human disease. In the pre-vitelliform stage, we saw a discrete disruption between the retinal pigment epithelium (RPE) and neural retina in the fovea-like region. This progressed to a yolk-like (vitelliform) manifestation, followed by the characteristic pseudohypopyon appearance, and finally the vitelliruptive (‘scrambled-egg’) stage.
Even more surprising were the morphological alterations of the RPE apical surface in BEST1mutant dogs, and the retina-wide microdetachment in areas that were otherwise normal. Further studies revealed retina-wide abnormalities at the RPE-photoreceptor interface associated with defects in the RPE apical microvillar ensheathment as the cause of the diffuse microdetachment. Completely unexpected was the substantial expansion of the microdetachment with light exposure over a matter of minutes.
How do you plan to translate your findings into treating humans?
Our labs have longstanding experience in developing successful treatments in dogs and bringing them to the clinic. Previous such examples include the RPE65 form of Leber congenital amaurosis, CNGB3 form of achromatopsia, and RPGR form of retinitis pigmentosa. For BEST1disease, our next step is to evaluate the safety of the gene augmentation therapy and identify the stages that are sensible to approach with therapy in bestrophinopathy patients. In view of anatomical similarities between dog and human eyes, and common surgical intervention techniques used, this could be completed within the next two years.
What’s next?
We’d like to address the question of whether the therapeutic window of the gene therapy treatment will extend to the later disease stages. Also, as our recently published work was on understanding and treating canine macular degeneration due to recessive mutations in BEST1, we are very interested in understanding the dominant disease by studying dogs and patients carrying heterozygous BEST1 mutations.
- KE Guziewicz et al., “BEST1 gene therapy corrects a diffuse retina-wide microdetachment modulated by light exposure”, Proc Natl Acad Sci USA, [Epub ahead of print], 2018. PMID: 29507198.
Following my journey through academia, I entered the world of scientific writing and never looked back. After several years of working as a medical writer – where I developed a wide range of skills in healthcare publications and communications – I took the opportunity to stretch my creative and journalistic muscles and joined Texere Publishing. Working as Editor on The Ophthalmologist allows me to nurture my skills in scientific writing – and explore the dynamic world of ophthalmology – all within an innovative and exciting company.















