Sonic Scalpel
In July 2016, INSIGHTEC received FDA approval for the first MR-guided focused ultrasound device to treat essential tremor. But could the noninvasive tool find wider utility as a safer alternative in other brain surgery procedures – or surgery in general? Eyal Zadicario shares the story and the science behind Exablate Neuro.
How did you get involved in the field of therapeutic ultrasound?
I have been with INSIGHTEC since it was founded in 1999. The company brought together a group of engineers and scientists who shared a vision of a breakthrough surgical tool that was not only noninvasive but also allowed the physician to monitor and optimize treatment in real time.
Ultrasound is essentially a wave of pressure that travels through tissue. Under certain parameters, this pressure wave is absorbed in the tissue and transformed into heat – when the temperature exceeds a critical point, the proteins in the tissue are denatured and the tissue dies, a process known as thermal ablation.
We knew that focused ultrasound had the ability to penetrate deep into the body, while having a very sharp focal effect, operating with sub-millimeter sharpness and millimeter accuracy. In fact, work had been going on for decades into therapeutic application of ultrasound, with some success. But targeting was not always as accurate, and effects not as reproducible, as required for a surgical tool.
How did you tackle the problem of precision?
To turn it into a repeatable, durable, and sustainable procedure, we needed to provide a means to guide this “virtual scalpel” to the target and get feedback on how the tissue responds during treatment. The solution we came up with was to link high-energy focused ultrasound technology to the most advanced imaging method available – MRI – to create MR-guided focused ultrasound (MRgFUS).
After several years spent developing the technology, we gained approval for the device to be used to treat uterine fibroids and painful bone lesions in metastatic cancer. But we always felt that the Holy Grail was operating noninvasively in the brain. The common understanding at the time was that ultrasound would not penetrate the bony structure of the skull. We have devoted almost two decades to developing the hardware and software needed to achieve that goal, and have conducted a series of clinical trials.
Why target the brain?
There is a major clinical need for a noninvasive alternative to surgery in this area. It’s not a coincidence that “brain surgery” is used as shorthand for a difficult, detailed task – it requires extreme accuracy, delicacy, and precision. You don’t want to expose the brain to any collateral effects, however small. There are also the risks that come with any invasive surgery – infection, side effects from general anesthetic, pain around the wound site, and so on.
Radiation therapy offers a noninvasive option for certain conditions, but it takes weeks or months for the tissue to be destroyed. The beauty of thermal ablation is that the tissue is destroyed as soon as it reaches a specific temperature. That means that the physician and patient can see right there and then whether it has been effective – and there is little risk of delayed side effects.
What were the challenges of working in the brain?
We realized early on that predictability was the crucial hurdle to overcome. Experiments with focused ultrasound in the brain had been going on for some time but there were several missing elements to make it a viable medical device. For a start, variability between patients meant there was no way to accurately predict what ultrasound parameters would generate the right level of heat in the right location. With the real-time monitoring that MRI provides, we were able to resolve that problem.
The other major challenge was getting the ultrasound waves into the brain in the first place. Many types of ultrasound are completely blocked by the dense tissue of the skull. Plus, the thickness of the skull is highly variable, making it hard to focus the ultrasound to the target. We developed hardware that can produce ultrasound powerful enough to penetrate the skull, and sophisticated software that can correct for skull shape and thickness.
The first clinical trials targeting the brain started in 2005. After several feasibility cases to prove the safety of the device, the FDA approved the pivotal study for treating essential tremor. There is a lack of good therapies for these patients and the FDA was very supportive, but it was a long, thorough process.
And 10 years on, you now have FDA approval…
The device, Exablate Neuro, has now been approved in Europe and the US for the treatment of essential tremor. It’s a common condition that causes uncontrollable shaking, most commonly in the hands. Medication often comes with serious side effects and isn’t always effective.
It has been known for decades that tremor can be controlled by disrupting the Vim nucleus of the thalamus. The problem is that it’s a tiny focal point (3-4 mm diameter), located deep in the brain, and surrounded by several vitally important neural networks. Currently, the most common approach to reach this target is deep brain stimulation, which involves implanting an electrode into the thalamus, powered by a controller placed under the skin on the chest. It can effectively suppress tremor, but carries the same risks as any brain surgery and requires regular follow-up and battery replacements. Not surprisingly, many essential tremor patients choose not have this invasive surgery, instead deciding to live with their symptoms. Now, focused ultrasound treatment offers them an alternative.
How does essential tremor affect patients’ lives?
The patients who are treated with our device have typically suffered for most of their life with essential tremor in their hands. Over time, their condition worsens, until they may have difficulty with everyday tasks. They can’t hold a glass of water. They can’t eat unaided. They can’t write. They can’t drive. They can’t do anything that involves delicate motor action. At rest, the tremor may be invisible, but as soon as they try to move their hands, it escalates, and the more delicate the movement they try to make, the more pronounced the tremor. Many are forced to stop working, and may be embarrassed to go out in public or eat with their family – it is very limiting.
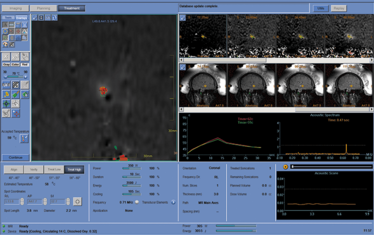
Exablate monitor showing thermal dose at target during treatment.
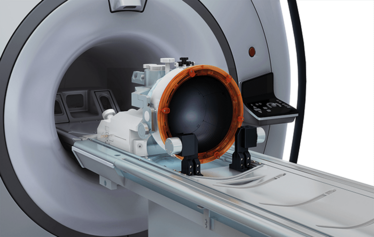
Exablate Neuro treatment bed inside the MRI.
What does treatment with Exablate Neuro look like?
This is a single-session treatment and we treat the tremor in the dominant hand. The patient – fully conscious – enters the MRI scanner, which is equipped with the focused ultrasound device. The physician views images from the MRI, marks the target on screen, and the focused ultrasound is directed to that target. The patient is completely awake during the procedure, and can be evaluated as the tremor improves and for any side effects. The physician will increase the temperature gradually, while monitoring the patient – if the patient starts to show signs of off-target effects, the treatment is adjusted before reaching a therapeutic temperature.
The patient spends around three hours in the MRI machine, and most walk away with their tremor significantly improved. Often, patients get very emotional – they may not have been able to eat or drink normally for many years, and then suddenly the tremor is gone in a few hours. It is very rewarding to see the impact on patients’ lives. It has been a very long, challenging technical development process, and seeing patients benefit from what is now 17 years of work is what keeps us all inspired.
There are now over 30 centers using the Exablate Neuro for both research and clinical use, with over 600 patients treated worldwide. We see this as the first test of taking the technology into the brain.
What’s next?
At the moment, we treat the dominant hand only, and patients with tremor in both hands are already asking why they cannot have the other hand treated too, so our immediate priority is to evaluate the possibility of bilateral treatment. We are also looking at other neurological causes of tremor, such as Parkinson’s disease. After that, we plan to turn our attention to other brain disorders that are currently treated with conventional surgery, such as tumors or epilepsy.
In the longer term, this technology has much more potential. There are two things we’re focusing on: one is thermal ablation for prostate, liver, and pancreatic cancer. The other is to enhance drug delivery to the brain. Drug delivery is quite a different challenge to ablation. Here, we use much lower power and a different frequency of ultrasound, so that the pressure wave is not absorbed and causes no heat, but simply vibrates the tissue structures and membranes. Preclinical trials have demonstrated that if you inject microbubbles into the bloodstream and apply the ultrasound field to certain areas of the brain, the vibrations increase the permeability of the blood–brain barrier and allow the microbubbles to enter. That is the concept, and we’re now looking at how to get that into a well-designed clinical trial. There are many therapies that could have potential to treat neurological conditions, but are currently unusable, since they are unable to cross the blood–brain barrier.
There is a lot of excitement about the potential of therapeutic ultrasound at the moment. We hope that the first approval for a neurosurgical indication will push the whole field forwards.
Eyal Zadicario is General Manager at INSIGHTEC.
Eyal Zadicario is the General Manager at INSIGHTEC















