Cancer Control on a Shoestring
As cancer rates continue to climb, low and middle income countries are ramping up prevention and screening efforts.
The burden of cancer in low and middle income countries (LMICs) is significant and growing year by year, accounting for around 70 percent of all cancer deaths worldwide. Cancer is increasing in LMICs for two main reasons. One is simple demography – populations are aging and when people live longer they are more likely to develop cancer. But risk factors are also changing. Incidence of cervical cancer is slowly falling in some countries, while breast cancer rates are way up – likely a consequence of changing patterns of reproduction. So-called “Western” lifestyles with limited physical activity and high levels of processed food are also coming into play.
In high-income countries, most cancer patients now survive for years after diagnosis, whereas in LMICs, less than a third of patients with cancer survive (1). Some cancers with a poor prognosis, such as lung, esophagus, stomach, and liver cancers, are more common in LMICs (2). And patients in LMICs are diagnosed much later, on average, than those in high-income countries.
As a clinical oncologist practicing in India during the 1980s, most of my patients came to me too late, when there was little to offer beyond palliative care. Those experiences made me determined to improve cancer control in India and other LMICs. Since the early 1990s, I have been pursuing that goal at WHO’s International Agency for Research on Cancer (IARC).
The IARC provides a platform for collaboration at an international level; individual countries and researchers can benefit from each other’s experiences, which is particularly important when we need to make the best use of limited resources. Working across so many countries gives us an overview of the global situation – and the chance to really influence public health policy and implementation.
My work focuses on early detection interventions related to major cancers, such as breast, cervix, colorectal and oral cancers, most of which are increasing in incidence. Some cancers lack good treatment options, but in many cases well established interventions exist but are not available or affordable for LMICs. I’m interested in learning how we can rapidly scale-up interventions and make them feasible in health services with limited resources.
Recently, the WHO has spearheaded a major focus on controlling non-communicable diseases, including cancer, diabetes, cardiovascular disease, and stroke. In 2012, WHO member states agreed on the goal of reducing premature death from non-communicable diseases by 25 percent by 2025, starting from a 2008 baseline. The United Nations (UN) Sustainable Development Goals for 2030 set a target to reduce premature deaths from non-communicable diseases by a third. A substantial number of those premature deaths are a result of cancer. It is an important opportunity for cancer control.
Two decades ago, such targets would have been impossible. Today, we see increasing awareness among government authorities about cancer prevention programs, and the picture is now far more optimistic than when I started out in this field.

A breast cancer awareness program in India.
A tale of two vaccines
Human papilloma virus (HPV) vaccination programs have been a great example of this new energy. HPV vaccination has already been rolled out in 80 countries (including 35 LMICs), with another 25 countries currently carrying out pilot studies. In stark contrast, the first anticancer vaccine (against hepatitis B) was first approved in the early 1980s, but took 20 years to be widely adopted. Only after it became a Gavi-eligible vaccine in 2000 – dramatically reducing the cost to health systems – did hepatitis B vaccination take off; by 2004, half of LMICs had introduced the vaccine. On the other hand, the HPV vaccine was made Gavi-eligible in 2013, six years after it was introduced.
South and Central American countries, where rates of cervical cancer have historically been some of the highest in the world, have been particularly quick to implement vaccination programs. In continental South America, almost all countries have introduced a national HPV vaccination program, and Central America is not far behind. However, other regions have faced barriers to implementation. More women die of cervical cancer in Asia than anywhere else in the world, but HPV vaccination has not yet gained momentum in the region. India has seen substantial misinformation about the safety of the vaccine, and any plans to introduce the vaccine have been put on hold. Japan introduced the vaccine and saw good uptake initially, but media reports of unfounded links between vaccination, and long-term pain and numbness in very few vaccinated girls pushed the government to withdraw their recommendation.
These experiences highlight the importance of public education. Malaysia is a notable exception within Asia, and provides a model for other countries to follow. Here, a comprehensive four-year education campaign prepared the public, schools and religious establishment for the introduction of the vaccine, and contributed to a very high rate of coverage.
Cancer Control Around the World
Lung
No national cancer control strategy is complete without an effective tobacco control program. The WHO Framework Convention on Tobacco Control is the first global public health treaty on the subject – signed by 180 countries – and provides a framework for countries without specific drug control policies. We know what works in tobacco control – sustained funding of comprehensive programs, tax increases on tobacco products, smoke-free policies and aggressive media campaigns. Now, we need to empower nations to implement these measures. Amongst all these interventions, taxation is probably the most effective. A ten percent increase in price reduces consumption of cigarettes by five percent in LMICs (3).
Breast
Breast cancer is the number one cancer of women in most countries. In developing countries, incidence is increasing by 1–3 percent per annum. At the moment, we lack specific prevention measures for breast cancer, but screening and breast awareness programs can help detect tumors at an earlier stage. Regular, systematic self-examination is often promoted, but a Chinese study involving over 266,000 women found no significant impact on mortality (4), and scientists are still addressing the question of whether a systematic clinical breast examination screening of asymptomatic women is more effective than general breast awareness. The greatest unmet need is in sub-Saharan Africa, where around 70 percent of women with breast cancer present with tumors larger than 5 cm (5).
Colorectal
Colorectal cancer is increasing at a rate of 1–2 percent per year in many developing countries. A pilot trial introducing fecal occult blood testing into primary care services in a province of Thailand showed significant detection rates for colorectal cancer (6) and led the Thai government to expand the scheme to another five provinces.
Liver
Thanks to investments in national immunization programs, and improvements in cold chain and capacity, there was a substantial improvement in LMIC hepatitis B vaccination rates between 2000 and 2012. Thailand was one of the first countries to incorporate hepatitis B vaccination in the national immunization program, in a phased introduction starting with pilot trials from 1988 onwards, and a recent study confirms that children born after the vaccine became standard are significantly less likely to be carriers (7). A 69 percent reduction in liver cancer in vaccinated young people have been reported from Taiwan, which introduced hepatitis B vaccination during 1984–86 (8).
Oral
Most cases are associated with tobacco and alcohol use so, as with lung cancer, substance control is vital. A 34 percent reduction in oral cancer mortality was seen following regular screening among users of tobacco or alcohol or both in a randomized trial in India (9). However, only two regions currently have oral cancer screening programs: Cuba and Taiwan.
A balancing act
There is still much to do. Over the next few years, IARC will be working to help increase the incorporation of HPV vaccines into national immunization programs. We also hope to see countries introducing cervical cancer screening and treatment for pre-cancer within their services, as well as increasing access to early diagnosis and treatment of breast cancer. Looking a little further ahead, we hope to see the introduction of more early detection programs in the primary healthcare services for colorectal cancer.
What is needed to make it happen? A stable budget to fund long-term programs is a prerequisite, along with adequate equipment and infrastructure, but it’s important to remember that money alone is not enough. One of the biggest bottlenecks at the moment in areas like sub-Saharan Africa is a lack of human resources. Not just doctors and nurses, but pathologists, epidemiologists, surgeons and technicians. Investments in hospitals and equipment are useless unless you also fund recruitment and training of healthcare workers.
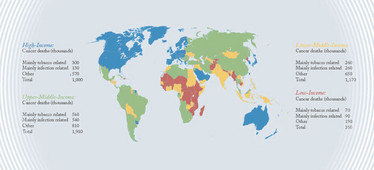
Cancer mortality before age 70 years, by World Bank income groupings, 2012.
Screening programs require a considerable investment in infrastructure and human resources. Cancer screening is something you have to do repeatedly, and by definition involves apparently healthy people. The introduction of a new screening program should go hand in hand with good educational awareness campaigns, to encourage participation. It is also important to apply a high level of quality assurance, by assessing false-positive tests and over-diagnosis, to make sure the program isn’t doing more harm than good. Evidence from successful programs in Europe and Australia suggests that a screening program takes at least 15–20 years to reach the target level of participation and start to show results.
On the other hand, we must remember that funding is a delicate balancing act. Prevention and screening are vital to reduce mortality in the long term, but for immediate impact, it’s important to invest in better diagnosis and treatment too. You do not need to have sophisticated infrastructure and approaches to detect and treat disease early; even with very basic interventions, you can make considerable inroads. The WHO has identified the “best buys” for LMICs in non-communicable disease prevention, including some specific to cancer. Tobacco control interventions, hepatitis B vaccination and some form of screening for precancerous cervical lesions are all interventions with impressive cost-effectiveness.
Having worked in clinical oncology, I am a passionate advocate for improving cancer services, but I recognize that there are many other healthcare needs and, increasingly, cancer control is being incorporated into wider non-communicable disease programs. We live in a changing world, with shifting priorities, and we have to be pragmatic about our place within those priorities.
Overall, cancer control in LMICs is improving, albeit slowly. Increased awareness and willingness from governments to introduce new interventions and improve existing services has led to huge strides over the past two decades, and I am hopeful for a future when everyone at risk of, or diagnosed with, cancer can expect a good standard of care.
Rengaswamy Sankaranarayanan is Special Advisor, Cancer Control and Group Head of the Screening Group at the WHO International Agency for Research on Cancer, Lyon, France.
- J Ferlay et al., “Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012”, Int J Cancer 136, E359–E386 (2015).
- Bray F, Soerjomataram I. “The Changing Global Burden of Cancer”, In: Disease Control Priorities: Volume 3, Cancer, edited by Gelband H, Jha P, Sankaranarayanan R, Horton S, (Editors), 3rd ed. World Bank, Washington, DC, USA (2015).
- P Jha, FJ Chaloupka, “Curbing the Epidemic: Governments and the Economics of Tobacco Control”, World Bank Publications; Washington, DC, USA (1999).
- DB Thomas et al., “Randomized trial of breast self-examination in Shanghai: final results”, J Natl Cancer Inst, 94, 1445–1457 (2002).
- F Islami et al., “Tumor size and stage of breast cancer in Côte d'Ivoire and Republic of Congo – Results from population-based cancer registries”, Breast 24, 713–717 (2015).
- T Khuhaprema, “Organised colorectal cancer screening in Lampang Province, Thailand: preliminary results from a pilot implementation programme”, BMJ Open 4:e003671 (2014).
- N. Posuwan et al., “The success of a universal hepatitis B immunization program as part of Thailand’s EPI after 22 years' implementation”, PLoS One. 11, e0150499 (2016).
- M.H.Chang et al., “Descreased incidence of hepatocellular carcinoma in hepatitis B vaccines: a 20- year follow-up study” J Natl Cancer Inst 101, 1348-1355 (2009).
- R. Sankaranarayanan et al., “Effect of screening on oral cancer mortality in Kerala, India: a cluster-randomized controlled trial”, Lancet 365, 1927-1933 (2005) .
Rengaswamy Sankaranarayanan is the Special Advisor of Cancer Control and Group Head of the Screening Group at the WHO International Agency for Research on Cancer (IARC).















