Buried Treasure
Noncoding DNA makes up the majority of our genomes, much of it being conserved and transcribed. Though the functions of small regulatory RNA molecules are well known, what about so-called long non-coding RNA? Pioneering scientists are digging up secrets hidden in the labyrinthine depths of our chromosomes.
Genomic explorations have offered us a great deal of insight into the complexities and intricacies of life. But for all the knowledge we’ve gained, there is still much we do not understand when we step outside the central dogma of “DNA to RNA to protein”. Take non-protein coding transcripts of over 200 nucleotides as a prime example. Previously thought to be “junk” genetic elements, new functions and classes of long non-coding RNAs (lncRNAs) are rapidly emerging.
A recent paper in Nature may raise the profile of the field even higher; researchers discovered that the SAMMSON lncRNA is expressed with exquisite specificity in human melanoma cells. And beyond that, SAMMSON is necessary for melanoma cell survival, making it a prime therapeutic target (1). SAMMSON stands for Survival Associated Mitochondrial Melanoma Specific ONcogenic, and refers to the biblical figure Samson. Just as Samson relied on his hair for his power, melanoma cells rely on SAMMSON for their survival.
“It’s a beautiful example of a hypothesis proving true, which is always very rewarding as a scientist. But the most exciting thing is definitely the huge potential, not only for this particular finding – SAMMSON for melanoma – but the mere idea that there could be hundreds of other lncRNAs out there with equally specific expression profiles, meaning that they could serve as diagnostic and therapeutic targets for other diseases,” says Jo Vandesompele, co-author and Professor in the Functional Cancer Genomics and Applied Bioinformatics (FCGAB) lab at Ghent University.
Pieter Mestdagh, co-lead researcher and Professor in the FCGAB lab, agrees, “As a cancer research lab, lncRNAs are a very exciting aspect for us to focus on. We are continuously looking for novel ways to diagnose and treat cancer, and we believe that the field of lncRNAs could be a game-changer.”

Pieter Mestdagh (left) and Jo Vandesompele. Credit: Ghent University.
One man’s trash…
The FCGAB lab uses high-throughput technology and advanced bioinformatics to hone in on RNAs linked to cancer. Vandesompele explains, “Our lab has done a lot of work this past decade on non-coding RNA. We started off looking at miRNA, before moving into the exciting new field of lncRNA – our ultimate goal is always the identification of therapeutic targets. And the very specific expression profiles of lncRNAs open up a number of opportunities in terms of therapeutic and diagnostic applications,” says Vandesompele.
In the past, lncRNAs were largely dismissed as genetic noise – or “junk” – and research into their potential functions only began in earnest about a decade ago. Since then, the relevance of lncRNA in cell homeostasis and disease mechanisms has become apparent. And though the field is still in its infancy, enthusiasm is growing quickly as the new functions of lncRNA are unraveled. “I think it was very unfair to call it junk, simply because we didn’t know its function. In school, we all learned the central dogma of biology: DNA is transcribed into RNA, and RNA into protein. But actually it turns out that just a minority of RNA does that. It’s evident now that the most important function of RNA is not to code into proteins, but to act as ‘glue’ that facilitates all kinds of biochemical processes. It’s a completely underappreciated functionality of human cells, and that’s part of the reason we were drawn to it,” says Vandesompele.
Mestdagh says he has been excited to witness the explosion of studies in the field. “When we started the study that led to the paper in Nature, there were only around 1700 lncRNAs listed in public databases. We were able to include them all in a single study. Now there are at least 60,000 known to be expressed in various human cell types.”
In that original study, the team were not looking specifically for potential melanoma drugs. They set out to investigate the differential expression of lncRNAs across different cancer types by profiling their expression in a panel of cancer cells. Looking at the expression profiles, there was one association that stood out. “When we started profiling the expression of these lncRNAs in cancer cells, we noticed that some of the lncRNAs were specifically expressed in only one cancer type,” says Mestdagh. “It was really a matter of letting the data speak for itself, and the most specific gene in the cohort was SAMMSON.”
The strength of SAMMSON
After profiling numerous normal and cancerous tissues, the researchers concluded that SAMMSON expression is highly specific to melanoma cells. Realizing that the nucleotide could have diagnostic or therapeutic possibilities, they decided to focus their ongoing investigation solely on SAMMSON. “It was Pieter who looked at the data and said ‘Wow, that could be indicative of a major survival function for melanoma cells – let’s try to silence that gene to find out how crucial it really is’,” says Vandesompele.
The team contacted Jean-Christophe Marine (co-lead researcher on the paper, and head of the VIB Laboratory for Molecular Cancer Biology at KU Leuven) to help confirm their hypothesis that SAMMSON was an oncogene. Mestdagh adds, “We are not melanoma experts, so we worked with the group at KU Leuven because they had prior experience with melanoma, and had model systems in place to start studying it.”
When the results of the VIB lab’s analysis came back, Vandesompele and Mestdagh were surprised by how completely dependent on SAMMSON the cancer was. “Silencing of SAMMSON caused melanoma cells to die very rapidly and very efficiently. We hypothesized that SAMMSON would have an important role, but we didn’t realize its effect on the cells would be so strong,” says Mestdagh. The same result was seen in various melanoma cell cultures, including those resistant to an existing therapy, dabrafenib.
To study the effects in more detail, the researchers used GapmeRs (antisense oligonucleotides that inhibit lncRNA function) to knock down SAMMSON, which allowed them to investigate the pathways that SAMMSON was involved with in melanoma. They pinpointed a key function in mitochondria, and further research led to the conclusion that silencing SAMMSON causes apoptosis in part by disrupting p32-mitochondrial functions, which are vital for the organelle’s homeostasis. The result is toxic over-accumulation of mitochondrial precursors in the cytosol, which eventually triggers cell cycle checkpoints or induces cell death, depending on the status of the cell.
Spinning Out
As well as working on long noncoding RNAs at Ghent University, Jo Vandesompele and Pieter Mestdagh are also involved in university spin-out Biogazelle, co-founded by Vandesompele and colleague Jan Hellemans in 2007. Mestdagh is a consultant/senior scientist at the biotech, which investigates the coding and non-coding regions of the genome. Biogazelle uses the technology developed at the Ghent lab, but at a larger scale. The company offers RNA biomarker discovery and development services, and biostatistical qPCR data analysis software to pharmaceutical customers. Biogazelle also has its own therapeutic program, focusing on blocking cancer-promoting lncRNAs with nucleic acid-based drugs.
Vandesompele is also the co-founder of another Ghent University spin-off company, pxlence, which provides a catalogue of almost a million PCR assays for targeted resequencing of exons and protein-coding genes.
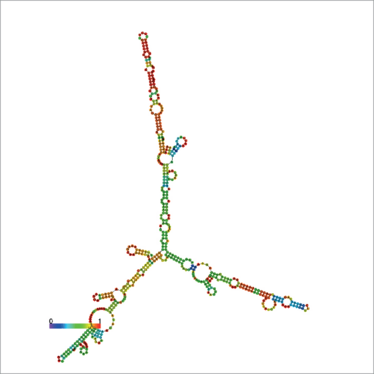
SAMMSON transcript
Mighty in mice
The researchers next analyzed the therapeutic potential of SAMMSON knockdown in vivo, using patient-derived xenografts of melanocytes in mice. They found that treatment with GapmeR3 to block SAMMSON expression decreased proliferation and increased apoptosis of tumor cells, and the results were enhanced when GapmeR3 was combined with BRAF inhibitor dabrafenib. Notably, combination treatment with GapmeR3 and dabrafenib didn’t cause any severe adverse effects or weight loss in the mice, unlike combinations of dabrafenib with a MEK inhibitor, trametinib.
The results suggest that SAMMSON knockdown could have a synergistic effect with existing cancer drugs – an important finding given that combination therapies are increasingly becoming the norm for cancer treatment.
“We’re definitely not claiming that SAMMSON-targeted therapy would be a single magic bullet. I think it’s clear that treating a devastating disease like malignant skin cancer requires combination therapy. But the addition of anti-SAMMSON treatments to other molecular targeting drugs could be a major step forward,” Mestdagh says.
The team is actively pursuing the therapeutic potential of anti-SAMMSON therapy. “We set up a collaboration with a major pharmaceutical company that has a lot of expertise in antisense technology, to explore the toxicity of antisense oligonucleotides directed to SAMMSON. These studies will be initiated in mice very soon, with the goal of bringing us one step closer to the clinical space,” says Mestdagh.
The researchers have also been pursuing an alternative avenue to silence SAMMSON. Small molecule drugs are still the therapy of choice for most pharmaceutical companies, and have a well-trodden route to the clinic. With that in mind, the FCGAB lab initiated a collaboration with Matthew Disney at the Scripps Research Institute in Florida to identify small molecule compounds that actively bind to the transcript and disrupt its function. “If successful, it could be the first small molecule targeting a lncRNA to treat cancer,” says Vandesompele.
SAMMSON’s abundant expression in melanoma cells and absence in normal cells could also make it a perfect candidate for diagnostic or prognostic tools. To that end, the team is currently evaluating whether SAMMSON is circulating in the bloodstream – and, if so, to what extent it could be used as a diagnostic or predictive marker.
Further research into SAMMSON expression has revealed that it is found in melanomas of the eye too. Uveal melanoma is the most common form of non-skin melanoma, and the most common eye cancer of adult Caucasians (about 2000 cases per year in North America). Compared with melanomas in the skin, which can be treated with BRAF and MEK inhibitors (although resistance develops rapidly in most cases), uveal melanomas are much more difficult to treat.
Mestdagh explains, “Metastatic uveal melanoma has virtually no effective treatment, with median patient survival times of less than one year. It’s a rare but deadly disease, and we hope we can make a difference. We still need to carry out a lot of experiments to prove that we can kill uveal melanoma cells in vivo, but in vitro results of SAMMSON inhibition have proven promising.”
Unanswered questions
Though SAMMSON looks promising as a therapeutic or diagnostic target, much is still unknown, providing a rich seam of future research for the FCGAB team. “We do see occasional expression of SAMMSON in non-melanoma cancer cells. It’s neither highly nor consistently expressed in these cells, but we’re following up with further studies to see whether it has similar roles in these rare cases where it is expressed. Then of course, the question becomes: why is it sometimes expressed and other times not? We have so much more to do, and so many research questions regarding SAMMSON still to investigate,” says Vandesompele.
Part of those investigations will involve attempting to unravel the relationship between SAMMSON and known oncogene MITF, its near-neighbor on the chromosome. MITF and the protein it encodes have a clearly established role in melanoma, so its close proximity seems unlikely to be a coincidence. However, the two genes do not appear to regulate each other, which has left the team puzzled. “It calls for further research to find out if it’s really a coincidence or if there is something that we are missing,” admits Vandesompele.
The lab continues to investigate the wider role of lncRNAs in cancer, and they hope their work may drive other researchers to enter a fascinating field and discover translational applications.
“I hope this will inspire colleagues and other researchers to really dig into the lncRNA domain, because there are so many genes that still need to be studied and so many functions that still need to be uncovered. If we can find other examples like SAMMSON that are crucial for cancer cell survival, cancer metastasis, or any process in cancer progression, it could lead to great results. We really need a large community of researchers interested in lncRNAs, because there’s a lot more work to be done,” concludes Mestdagh.
- E Leucci et al., “Melanoma addition to the long non-coding RNA SAMMSON”, Nature, 531, 518-522 (2016). PMID: 27008969.
My fascination with science, gaming, and writing led to my studying biology at university, while simultaneously working as an online games journalist. After university, I travelled across Europe, working on a novel and developing a game, before finding my way to Texere. As Associate Editor, I’m evolving my loves of science and writing, while continuing to pursue my passion for gaming and creative writing in a personal capacity.