Unlocking the Potential of Checkpoint Inhibition
Like many drugs today, pembrolizumab (Keytruda) was the result of a group effort. Here, we catch up with two scientists who shaped the destiny of the biotherapeutic at very different stages in its development.
At a Glance
International non-proprietary name (INN): Pembrolizumab
Brand name: Keytruda
Previous name: MK-3475, lambrolizumab
Developed by: Organon (acquired by Schering-Plough in 2007)
Marketed by: Merck & Co/MSD (merged with Schering-Plough in 2009)
Drug class: PD-1 inhibitor
Approval status: Approved in USA for advanced melanoma patients already treated with ipilimumab in September 2014 with an expanded indication granted in December 2015 for patients with unresectable or metastatic melanoma, and in October 2015 for treatment of patients with metastatic PD-L1-positive non-small cell lung cancer. In Europe, it has been recommended for the treatment of unresectable or metastatic melanoma since May 2015.
The Human Touch
David Matthews is associate director of biotherapeutics at MRC Technology. We spoke with Matthews to find out how MRC Technology helped the drug’s inventors take the first steps towards human trials.
Why is pembrolizumab important?
Firstly, it is active in hitherto very difficult-to-treat cancers, in some cases inducing complete remission, which is amazing. Secondly, it was the first successful PD-1 pathway inhibitor and one of the first checkpoint inhibitors. It’s a game-changing approach, and has opened our eyes to the possibility of targeting more cancers using similar mechanisms. The pharmaceutical industry is now putting a lot of investment and effort into understanding the pathways involved and hopefully coming up with more checkpoint inhibitors that can target more cancers.
How does PD-1 blockade lead to tumor regression?
There is a whole slew of molecules that are used to keep the immune system in check, to prevent inappropriate immune activation, which can lead to autoimmune disease. PD-1 is one such molecule. Found on T cells, when engaged it dampens the immune response to prevent immune cells attacking the body’s own tissues. Certain types of tumor cells take advantage of this mechanism by expressing a PD-1 ligand (PD-L1). When PD-L1 on a cancer cell binds to PD-1 receptor on a T cell, it prevents the immune system recognizing the tumor as abnormal. Blocking PD-1 binding with the pembrolizumab antibody means that T cells regain the ability to recognize the tumor. Put simply, pembrolizumab unmasks the tumor, so the body’s own immune defenses can recognize and destroy it. It’s not a panacea – not all cancers express PD L1 – but the hope is that other similar checkpoint inhibitors can be identified and blocked.
What is the overarching aim of MRC Technology?
We’re a nonprofit organization whose mission is to bridge the gap between academia and industry in developing new therapeutics. We team up with academics and biotechs to take care of the non-research side of drug development; for example, therapeutic antibody development, assay design and target validation. It isn’t the type of work that is very publishable, but it is a vital step in turning an idea into a candidate therapeutic. Essentially, together with a university or research institute we de-risk projects to make them more desirable for pharma to take on and develop into the clinic. MRC Technology develops small molecule and antibody drugs for a wide variety of diseases, but my team is focused on biotherapeutics.
What was your role in developing pembrolizumab?
We helped the original inventors at Organon create a humanized version of the antibody. Other people had worked on PD-1 antibodies before Organon, but unfortunately they never took them forward, otherwise drugs like pembrolizumab could have been in the clinic a decade ago. Organon had the confidence and determination to take it to the clinic but, as a small company, it lacked the skills and experience to humanize the antibody in-house, so they asked us to take on the job back in 2007. We have been carrying out antibody humanization since the early 1990s so we’re one of the most experienced groups out there - this is the fourth therapeutic antibody we have humanized to be approved for treating patients.
What were the challenges?
In this case the mouse and human genes were not a good fit – a fairly common occurrence but it meant we had to do some additional engineering. You can read published protocols on how to humanize antibodies, but they won’t tell you how to fix those types of problems. That’s where our know-how comes in. Assuming the antibody is potent enough, the next questions we ask focus on whether the antibody looks like a drug. Is it soluble? Does it aggregate? Is it stable at room temperature? We do a panel of biophysical assessments to make sure it has the properties needed for a viable drug and, if it makes the grade, it’s ready to go onto the next stage: preclinical development and manufacturing.
How did you feel when you heard pembrolizumab had been approved?
Seeing pembrolizumab on the market and having a truly life-changing impact for some patients made us feel very proud. Our part in the drug development process is a long way before the treatment reaches patients, but creating a drug that might help someone is why we do what we do, on a personal and organizational level. How many people can say that have been directly involved in creating something that saves lives?
Into the Clinic
Eric Rubin, vice president of oncology clinical research at Merck & Co, tells us how the company guided the drug through a trailblazing regulatory approvals process – and shares what’s next for cancer immunotherapies.
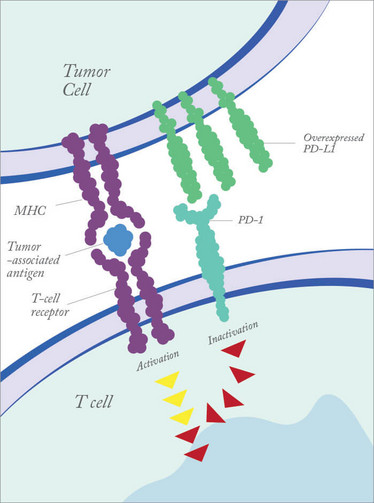
Figure 1. Tumor cells can dampen immune responses by overexpressing PD-L1.
When did you get involved in the development of pembrolizumab?
The drug came to us when we merged with Schering-Plough in 2009. I was part of the group tasked with examining the merged pipeline to decide how to prioritize our efforts. One of the compounds we selected at that time was the anti-PD-1 antibody MK3475, now known as pembrolizumab. That was around the beginning of what I would call a renaissance of immunotherapy in oncology, and data from trials of anti-CTLA-4 antibodies suggested that checkpoint inhibitors were promising targets, although there was a fair bit of toxicity associated with that particular target. We decided to start with a relatively small Phase I study of MK3475 about four years ago and it’s been an amazing journey since then. That study has now become a great example of rapid drug development. From the initial small trial, it grew to become a 1200-patient study, which is obviously very unusual for a first-in-human trial. The drug is now approved in patients with advanced melanoma and lung cancer, along with a companion diagnostic. For me, it has been a great example of collaboration between various stakeholders involved in drug development.
Not the typical regulatory path…
No. The typical purpose of a Phase I trial is assessing safety and dose finding. Many of them will have some efficacy component and our original protocol had a small number of patients with melanoma to try to get a sense of the efficacy. In the traditional approach, you would then go to a Phase II trial and ultimately a randomized Phase III trial. But in this case the dramatic results we saw right from the very beginning led us to seek an accelerated path. Luckily for us, around that time new legislation was enacted in the US to allow the FDA to designate breakthrough status to groundbreaking new therapies. Pembrolizumab was the first oncology drug to get breakthrough designation, which gave us the flexibility to grow the Phase I study and obtain enough information to demonstrate a positive risk–benefit ratio.
Was it tough being the first to take that path?
Being first is always challenging and we all had to learn as we went along. But actually, I think there are challenges for drugs awarded breakthrough designation now too. When Congress authorized this designation they did not authorize an increase in FDA staffing to deal with the additional workload. When there were only a handful of drugs in the pathway, it was relatively easy to access FDA officials. Now, there are dozens of drugs with breakthrough designation, so it is much harder for the FDA to live up to that expectation. There are a couple of bills that are working their way through the US government to try to increase resources at the FDA to maintain the efficiency of the accelerated approval process. In addition, I think the FDA is keen to make it clear that this route is intended only for true breakthrough drugs. It’s important that people understand that traditional randomized trials will still be required for the vast majority of new therapies. This was a nice demonstration of how it can be done, but it isn’t the expected path for every drug.
What’s next?
I’ve been lucky enough to meet people who were facing a guaranteed death sentence before being treated with pembrolizumab and are now approaching three years with no detectable cancer. It is wonderful to hear their stories, but it’s a sad fact that most patients won’t have such a good outcome – their cancer will eventually progress despite getting pembrolizumab. We’re working very hard to find ways to extend the benefits of the drug.
How do you hope to achieve that?
A very active area in cancer medicine right now is combination therapies. There are so many potential combinations with pembrolizumab, it’s hard to decide which ones to study first. We have nearly 80 clinical trials ongoing or planned with combinations of pembrolizumab and a variety of other drugs, including: other immune modulators, such as antagonist/agonist checkpoint inhibitors and vaccines; standard therapies, such as chemotherapy and radiation; and targeted therapies, such as kinase inhibitors. All look promising for some patient groups based on translational and preclinical work, and we’re excited about the possibilities.
Of course, you need to know which drug (or combination of drugs) to give to which patients. We already have a companion diagnostic on the market to help select lung cancer patients most likely to respond to pembrolizumab. Developed in collaboration with diagnostics company Dako, the test looks for the presence of PD-L1, one of the ligands for PD-1. Lung cancer patients with tumors expressing high levels of this protein have a response rate to pembrolizumab of 40 to 50 percent, compared with below 10 percent in patients with low expression. We’ll be conducting further studies of both PD-L1 and PD-L2 to find other ways to identify patients most likely to respond.
Vice President of Oncology Clinical Research at Merck & Co.
Associate Director of Biotherapeutics at MRC Technology.